Use of Tissue Morcellator in Laparoscopic Surgery
The morcellator is used to grasp the tissue to be removed and cuts it into small bits, which are forced into the hollow part of the instrument. It can be designed to remove a myoma or an ovary. It can be introduced through a 10 mm port or through the colpotomy wound.
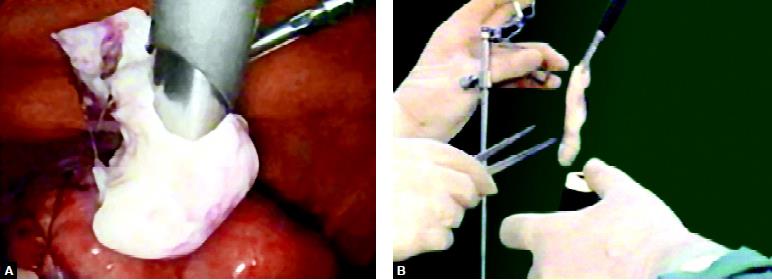
Morcellation of Myoma in Myomectomy
Tissue Morcellator and its Complication
The FDA currently estimates that a hidden uterine sarcoma may be present in approximately 1 in 225 to 1 in 580 women undergoing surgery for uterine fibroids based on recent publications. The FDA also estimates that a leiomyosarcoma may be present in approximately 1 in 495 to 1 in 1100 women undergoing surgery for uterine fibroids based on recent studies. Prior to 2014, the clinical community estimated uterine sarcomas to be present much less frequently, in as few as 1 in 10,000 women undergoing surgery for uterine fibroids. Several studies show that using a laparoscopic power morcellator during gynecological laparoscopic surgery in women with hidden uterine sarcomas is associated with lowering their chances of long-term survival without cancer. While these studies have limitations, women who have had fibroid surgery with a laparoscopic power morcellator later found to have a hidden uterine sarcoma, have lower disease-free survival, when compared to women who were treated with manual morcellation or without morcellation.

Uterine sarcomas and uterine fibroids may have similar signs and symptoms. At this time, there is no reliable method for predicting or testing whether a woman with fibroids may have a uterine sarcoma. The FDA recommends gynecologist share this information with patients and warns against using laparoscopic power morcellators in gynecologic surgeries to treat patients with suspected or confirmed cancer, and in women over age 50 having a myomectomy or hysterectomy for uterine fibroids.
Morcellator use with the tissue containment system
The FDA recommends that gynecologists use tissue containment systems when using laparoscopic power morcellators and that they ensure the laparoscopic power morcellator and tissue containment system are compatible. Legally marketed laparoscopic power morcellation containment systems are intended to isolate and contain tissue that is considered benign. Based on testing and clinical data, the use of a containment system confines morcellated tissue within the containment system.
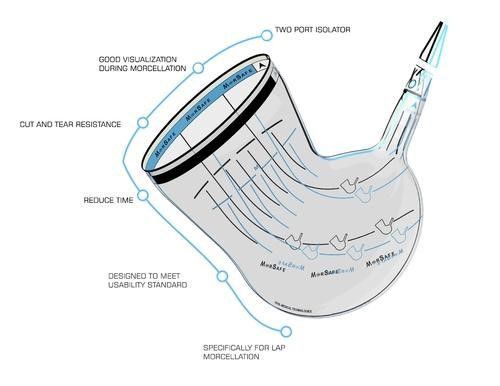
MorSafe tissue containment systems
Due to this increased risk, we continue to recommend the use of laparoscopic power morcellation only inappropriate women undergoing myomectomy or hysterectomy. In addition, it is good that when morcellation is appropriate, only contained morcellation be performed. MorSaf is an innovative single-use disposable device intended to be used as a receptacle for benign tissue mass during gynecological procedures such as laparoscopic myomectomy or laparoscopic hysterectomy. MorSaf is to be used in conjunction with the morcellator to safely contain and remove the shredded benign tissue mass. The device has unique features to allow for quick deployment, insufflation, morcellation, and spill-proof withdrawal of the bag. MorSafe, with its unique two-port design, offers the surgeon superior visibility during the surgery compared to a single port approach. Designed to fit and take the shape of the abdomen, it has been constructed utilizing a special tear-resistant material to prevent leakage. It also contains a special ring in the bag opening to allow the surgeon ultimate control of the bag opening and easy access to the interior of the bag during surgery.
The morcellator is used to grasp the tissue to be removed and cuts it into small bits, which are forced into the hollow part of the instrument. It can be designed to remove a myoma or an ovary. It can be introduced through a 10 mm port or through the colpotomy wound.

Morcellation of Myoma in Myomectomy
Tissue Morcellator and its Complication
The FDA currently estimates that a hidden uterine sarcoma may be present in approximately 1 in 225 to 1 in 580 women undergoing surgery for uterine fibroids based on recent publications. The FDA also estimates that a leiomyosarcoma may be present in approximately 1 in 495 to 1 in 1100 women undergoing surgery for uterine fibroids based on recent studies. Prior to 2014, the clinical community estimated uterine sarcomas to be present much less frequently, in as few as 1 in 10,000 women undergoing surgery for uterine fibroids. Several studies show that using a laparoscopic power morcellator during gynecological laparoscopic surgery in women with hidden uterine sarcomas is associated with lowering their chances of long-term survival without cancer. While these studies have limitations, women who have had fibroid surgery with a laparoscopic power morcellator later found to have a hidden uterine sarcoma, have lower disease-free survival, when compared to women who were treated with manual morcellation or without morcellation.

Uterine sarcomas and uterine fibroids may have similar signs and symptoms. At this time, there is no reliable method for predicting or testing whether a woman with fibroids may have a uterine sarcoma. The FDA recommends gynecologist share this information with patients and warns against using laparoscopic power morcellators in gynecologic surgeries to treat patients with suspected or confirmed cancer, and in women over age 50 having a myomectomy or hysterectomy for uterine fibroids.
Morcellator use with the tissue containment system
The FDA recommends that gynecologists use tissue containment systems when using laparoscopic power morcellators and that they ensure the laparoscopic power morcellator and tissue containment system are compatible. Legally marketed laparoscopic power morcellation containment systems are intended to isolate and contain tissue that is considered benign. Based on testing and clinical data, the use of a containment system confines morcellated tissue within the containment system.

MorSafe tissue containment systems
Due to this increased risk, we continue to recommend the use of laparoscopic power morcellation only inappropriate women undergoing myomectomy or hysterectomy. In addition, it is good that when morcellation is appropriate, only contained morcellation be performed. MorSaf is an innovative single-use disposable device intended to be used as a receptacle for benign tissue mass during gynecological procedures such as laparoscopic myomectomy or laparoscopic hysterectomy. MorSaf is to be used in conjunction with the morcellator to safely contain and remove the shredded benign tissue mass. The device has unique features to allow for quick deployment, insufflation, morcellation, and spill-proof withdrawal of the bag. MorSafe, with its unique two-port design, offers the surgeon superior visibility during the surgery compared to a single port approach. Designed to fit and take the shape of the abdomen, it has been constructed utilizing a special tear-resistant material to prevent leakage. It also contains a special ring in the bag opening to allow the surgeon ultimate control of the bag opening and easy access to the interior of the bag during surgery.





