Ureteral Injury and Laparoscopy
Ureteral Injuries
Ureteral injury is one of the most serious complications of gynecologic surgery. Ureteral injury during laparoscopic surgery has become more common as a result of the increased number of laparoscopic hysterectomies and retroperitoneal procedures that are being performed. Consequently, prevention of ureteral injuries should be a priority during laparoscopic gynecologic surgery. When a ureteral injury does occur, quick recognition of the problem and a working knowledge of its location and treatment are essential in providing patients with optimal medical care. Detailed anatomic knowledge of the retroperitoneum is necessary to prevent ureteral injuries.
The ureters are retroperitoneal tubular structures that extend from the renal pelvis, coursing medially and inferiorly to the bladder. Each ureter travels inferiorly along with the psoas muscle and crosses the iliac vessels at approximately the level of the bifurcation of the common iliac arteries. In females, the ureter is crossed anteriorly by the ovarian vessels as they enter the pelvis. Inferiorly, they are crossed anteriorly by the uterine artery. At this point, they enter the cardinal ligament, approximately 1.5 to 2.0 cm lateral to the cervix before their insertion into the trigone of the bladder.
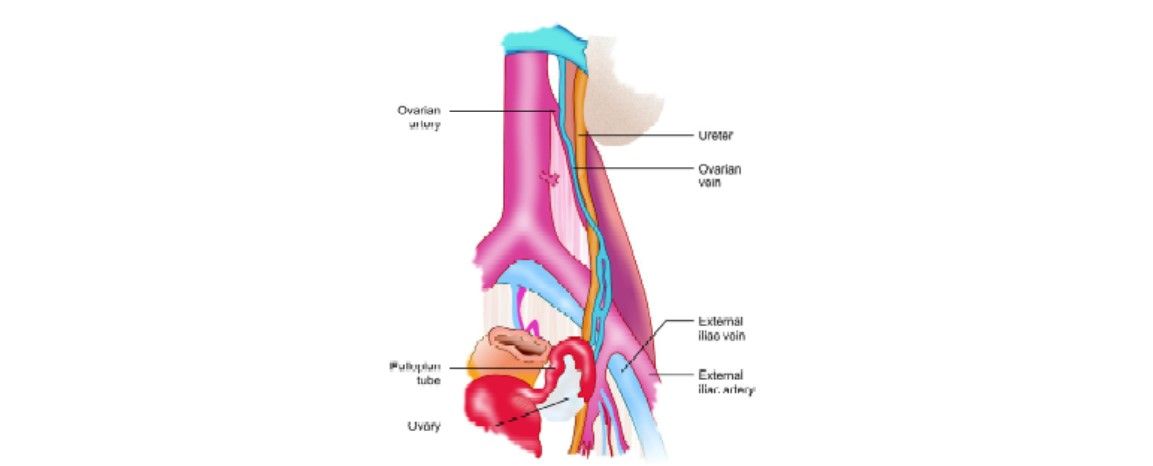
Anatomy of ureter
The ureters derive their blood supply from the renal artery, aorta, gonadal artery, and common iliac artery while they traverse intra-abdominally. These vessels approach the ureter from its medial side and course longitudinally within the periureteral adventitia. In the pelvis, the ureter derives its blood supply from the internal iliac artery or its branches. These vessels approach the ureter from its lateral side and also course longitudinally within the periureteral adventitia.
A significant ureteral injury is defined as any recognized or unrecognized iatrogenic trauma to the ureter that prevents it from functioning properly or effectively. The injury may lead to acute ureteral obstruction (e.g. a ureter that is inadvertently ligated) or discontinuity (i.e. inadvertent ureteral resection). If an injury to the ureter has occurred and is unrecognized, it may lead to chronic ureteral obstruction (i.e. crush injury, ischemia) or the formation of fistulas.
Frequency of Ureteral Injury
The frequency of ureteral injury following gynecologic surgery is approximately 1 percent, with a higher percentage of injuries occurring during abdominal hysterectomies and partial vaginectomies. Patients who have received pelvic radiation or who have advanced pelvic cancers requiring extensive surgical procedures are more likely to experience a ureteral injury.

Ureteric injury during laparoscopic hysterectomy
The rate of ureteral injuries in laparoscopic procedures varies. While some physicians report that laparoscopic procedures have an equivalent rate of ureteral stricture formation secondary to ureteral injury, other authors argue that the rate of ureteral strictures is significantly higher. More research is necessary before a definitive statement can be made regarding the rates of ureteral injury during laparoscopy.
Etiology
The six most common mechanisms of operative ureteral injury are as follows:
• Crushing from misapplication of a clamp
• Ligation with a suture
• Transection (partial or complete)
• Angulation of the ureter with secondary obstruction
• Ischemia from ureteral stripping or electrocoagulation
• Resection of a segment of ureter
• Excessive use of Monopoler which creates remote injury of the ureter.
Any combination of these injuries may occur.
Several predisposing factors have been identified in iatrogenic urologic injury. These factors include uterus size larger than 12 weeks gestation, ovarian cysts 4 cm or larger, endometriosis, pelvic inflammatory disease, prior intraabdominal operation, radiation therapy, an advanced state of malignancy, and anatomical anomalies of the urinary tract. Ureteral injuries can be either expected or unexpected, and they may be the result of carelessness or due to a technically challenging procedure.
Level of Ureteral Injuries
Intraoperative ureteral injury may result from transection, ligation, angulation, crush, ischemia, or resection.
There are three specific anatomic locations for potential ureteral injury during gynecologic laparoscopy:
• At the infundibulopelvic ligament
• At the ovarian fossa, and
• In the ureteral canal.
Among all the ureteral injuries 14.3 percent occurred at or above the level of the pelvic brim, 11.4 percent occurred at or above the uterine artery, and 8.6 percent occurred at the level of the bladder. The initial procedure in 20 percent of these cases was a laparoscopic-assisted vaginal hysterectomy. Alterations to normal anatomy may also hinder the identification of the ureters as in severe endometriosis, which may involve the ureter and also cause intraperitoneal adhesions.

Intravenous pyelogram (IVP) showing ureteric injury
Prevention of Ureteral Injury
Injury to the ureters can be prevented by meticulous surgical technique and adequate visualization.
Techniques to enhance visualization include:
• Ureteral catheterization with lighted stent: Ureteral catheterization with lighted stents have been used to assist in identifying the location of the ureters during laparoscopic surgery to help prevent iatrogenic injury. If the lighted stents are not visible during laparoscopic surgery, 4 options are available, as follows:
– Change the intensity of the laparoscopic lighting. By dimming the lights, the light from the stent may become visible.
– Change the camera to a different port.
– Identify the ureter where it is visible and follow it down to the surgical field.
– Convert to an open procedure so that the ureter can be palpated and identified.
Although ureteral catheterization helps to identify the ureters. However, in a large review of major gynecologic surgeries, Kuno et al. found that ureteral catheterization did not substantially reduce the risk of ureteral injury. The surgeon must practice meticulous
surgical technique and have intimate knowledge of the ureter's course to prevent ureteral injury.
• Hydrodissection: By making a small opening in the peritoneum and injecting 50 to 100 ml of lactated Ringer's or normal saline solution along the course of the ureter, one can displace the ureter laterally and create a safe plane within which to operate.
• Preoperative intravenous pyelogram: Intravenous pyelogram (IVP) has been used to locate the ureters in high-risk patients with potentially distorted anatomy; however, this did not decrease the risk of ureteral injury.
Recognition of Ureteral Injury
Once a ureteral injury is suspected, the ureter must be identified to assess the severity of the injury. A ureteral injury should be suspected with the presence of hematuria or urinary extravasation. Intravenous indigo carmine may be given to aid in the diagnosis and localization of the site of injury. Unfortunately, the majority of ureteral injuries are diagnosed in the postoperative period. Patients who present with postoperative fever, flank pain, and leukocytosis should undergo evaluation for ureteral injury.
Pathophysiology
The pathophysiology of ureteral injury depends on many factors, including the type of injury and when the injury is identified. Numerous consequences may occur after a ureteral injury, including spontaneous resolution and healing of the injured ureter, hydronephrosis, ureteral necrosis with urinary extravasation, ureteral stricture formation, and uremia.
Spontaneous Resolution and Healing
If the injury to the ureter is minor, easily reversible, and noticed immediately, the ureter may heal completely and without consequence. Inadvertent ligation of the ureter is an example of such an injury. If this injury is noticed in a timely fashion, the suture can be cut off the ureter without significant injury.
Hydronephrosis
If complete ligation of the ureter occurs, the urine from the ipsilateral kidney is prevented from draining into the bladder, leading to hydronephrosis and progressive deterioration of ipsilateral renal function. These events may occur with or without symptoms. If the urine in this obstructed system becomes infected, the patient will almost certainly become septic with pyonephrosis.
Ureteral Necrosis with Urinary Extravasation
In complete unrecognized ligation of the ureter, a section of the ureteral wall necrosis because of pressure-induced ischemia. The ischemic segment of the ureter eventually weakens, leading to urinary extravasation into the periureteral tissues. If the urinary extravasation drains into the adjacent peritoneum, urinary ascites may develop. If the urinary ascites is infected, peritonitis may ensue. If the peritoneum has remained closed, a urinoma may form in the retroperitoneum.
Ureteral Stricture
Ureteral stricture may occur when the adventitial layer of the ureter is stripped or electrocoagulation. When the adventitia, the outer layer of the ureter that contains the ureteral blood supply, is disturbed by either stripping or electrocoagulation, ischemia to a particular segment of the ureter may result. Ischemic strictures of the ureter may then develop, leading to obstruction and hydronephrosis of the ipsilateral kidney.
Uremia
Uremia results when a ureteral injury causes total urinary obstruction. This may result from bilateral ureteral injury or from a unilateral injury occurring in a solitarily functioning kidney. Anuria is the only immediate sign of imminent uremia. These cases require immediate intervention to preserve renal function.
Management
Depending on the type, duration, and location of the ureteral injury, surgical treatment may range from simple removal of a ligature to ureteroneocystostomy. The most common surgical treatments for ureteral injury are simple removal of a ligature, ureteral stenting, ureteral resection, and ureteroureterostomy, transureteroureterostomy, and ureteroneocystostomy.

Treatment logarithm of ureteric injuries
Observation
If a clamp or ligature constricting the ureter is discovered, the clamp or ligature should be removed immediately, and the ureter should be examined. If ureteral peristalsis is preserved and it is believed that minimal damage has occurred, the ureter injury may be managed with observation.
Ureteral Stenting with or without Ureterotomy
If tissue ischemia or partial transection of the ureteral wall is suspected, a ureteral stent should be placed. The purpose of the stent, which is typically placed cystoscopically, is to act as a structural backbone onto which the healing ureter may mold. It also guarantees drainage of urine from the renal pelvis directly to the urinary bladder. It also can work as a gentle dilator since it moves slightly in an up-and-down motion, associated with breathing, as the kidney unit moves. The use of the stent is thought to minimize the rate of obstruction of ureteral stricture in the injured area.
Alternatively, a ureterotomy may be made along the length of the injured or strictured section of ureter before the placement of a stent. Davis described this technique in 1943 (the Davis intubated ureterotomy) in which a ureterotomy is made and left open over the stent. The ureter eventually heals to form a watertight closure over the stent. The stent is withdrawn 6 weeks after it is placed, as it is estimated that all ureteral healing has occurred by that time.
The principles of the Davis intubated ureterotomy have been extended to endoscopic treatments of ureteral strictures. Ureteroscopic endoureterotomy and Acucise endoureterotomy are two modalities that are used to attempt to treat the segment of strictured ureter endoscopically by a longitudinal full-thickness ureteral incision, followed by stent placement. The success of these procedures closely resembles the success of the open Davis intubated ureterotomy, which approaches 80 percent patency at 3 years.
Ureteral Resection and Ureteroureterostomy
The establishment of an anastomosis between two ureters or between two segments of the same ureter. This end-to-end anastomosis between two portions of a transected ureter can be done by open as well as laparoscopic surgery. If extensive ischemia or necrosis is the result of an injury, the ureter injury is best treated by excising the injured segment of the ureter and re-establishing continuity with the urinary system. If the ureteral injury occurred above the pelvic brim, the simplest reconstruction is a ureteroureterostomy, a procedure that is indicated for injuries to short segments of the ureter (i.e. < 2 cm), in which an anastomosis is performed between the 2 cut edges of the ureter.
Transureteroureterostomy
Transureteroureterostomy (TUU) is a urinary reconstruction technique that is used to join one ureter to the other across the midline. It offers patients with distal ureteral obstruction an option to live without external urostomy appliances or internal urinary stents. TUU is also used in undiversion procedures when the surgeon wants to avoid the pelvis because of previous trauma, surgery, or radiation therapy.
If ureteroureterostomy cannot be performed technically and the defect is too proximal in the ureter for ureteroneocystostomy, transureteroureterostomy may be performed. Absolute contraindications to transureteroureterostomy include urothelial cancer, contralateral reflux, pelvic irradiation, retroperitoneal fibrosis, or chronic pyelonephritis. Stone disease, which was once considered an absolute contraindication, is now considered a relative contraindication by some urologists, based on the current ability to prevent stone formation in over 90 percent of patients with medical therapy.
Ureteroneocystostomy
An operation to implant the upper end of a transected ureter into the bladder. If the ureteral injury occurred below the pelvic brim, where visualization of the ureter is difficult and where the vesical pedicles overlie the ureter, ureteroureterostomy is often too difficult to perform. In these cases, 2 types of ureteroneocystostomy procedures are indicated, either a psoas hitch or a Boari flap, in which the bladder is mobilized to reach the easily identifiable ureter proximal to the injury. Boari flaps are contraindicated in patients with prior pelvic radiation, a history of bladder cancer, or any condition with a thick, hypertrophied bladder wall.
Preoperative Details
If consultation with a urologist is indicated intraoperatively, the urologist dictates no specific preoperative preparation. If a ureteral injury is identified after the patient is stabilized following the initial gynecologic operation, a discussion is conducted regarding the possible treatment options. Preoperative antibiotics that target urinary organisms should be administered. If patients are persistently febrile secondary to a potentially infected and obstructed renal unit, percutaneous nephrostomy on the affected side may be indicated. Pertinent radiographic studies (e.g. IVU, CT scan) may be used to help define the location of ureteral injury preoperatively.
Intraoperative Details
Ureteral Stent Placement with or without Ureterotomy
The perineum of the patient should be prepared and draped in the standard sterile manner and while the patient sedated adequately or anesthetized, a cystoscope should be inserted into the bladder.
After the bladder is examined and the ureteral orifices are identified, the ureteral orifice on the side of the injury should be cannulated with a ureteral catheter. A dilute cystografin-gentamicin mixture should be injected slowly through the ureteral catheter under fluoroscopy. Fluoroscopy should reveal the course of the ureter and identifying potential sites of injury.
A Teflon-coated guidewire should be placed under fluoroscopic guidance through the ureteral catheter and up the ureter into the renal pelvis. A double-J stent should be placed over the wire and pushed so that its proximal J-hook is placed within the renal pelvis and its distal J-hook is within the bladder. Then, the wire is pulled, and the stent position is reaffirmed fluoroscopically. The proper length of the stent can be estimated from the measured length of the ureter on retrograde pyelography from the ureteral orifice to the ureteropelvic junction. Allowing for roughly 10 percent magnification from the radiograph, subtract 2 to 3 cm, and select that length ureteral stent. If, after placement, the stent is not well-positioned because of inadequate or surplus length, it is best to replace it with a stent of proper dimensions.

Stent in the ureter
If an endoscopic ureterotomy is to be made, prior to placing the stent, retrograde pyelography should be performed to delineate the ureteral anatomy, and a Teflon- coated guidewire, acting as safety wire, is positioned into the renal pelvis and out through the urethra.
With ureteroscopic endoureterotomy, a rigid ureteroscope should be placed through the ureteral orifice and into the ureter lumen until the ureteral lesion can be visualized. The ureteral stricture is then cut with a probe from a number of cutting modalities, including Holmium laser or electrocautery. A full-thickness incision through the ureteral wall should be made until periureteral fat is visualized. Retrograde pyelography should be performed; extravasation of contrast outside the ureter should be seen. A wide-caliber ureteral stent should be then placed (usually 8F) in the fashion described above.
If Acucise endoureterotomy is performed, the Acucise device should be placed over the safety wire. Once a position is confirmed via fluoroscopic guidance and the orientation of the cut is set, the Acucise balloon is inflated and electrocautery is instituted. The Acucise device should be withdrawn, retrograde pyelography should be performed to confirm extravasation, and a wide-caliber ureteral stent should be placed in the fashion described above.
The formal Davis intubated ureterotomy is typically performed intraoperatively only when consultation with a urologist is called for while the patient is open. In this case, the injured ureter is cut sharply in a longitudinal fashion. A stent then can be placed to the kidney and bladder through the ureteral incision.
Ureteroureterostomy
The end-to-end anastomosis of the two portions of a transected ureter. If the urologist is asked to evaluate the ureteral lesion intraoperatively, further dissection of the existing exposure is often necessary, because the lack of exposure is the most likely contributor to the injury. Additional blunt and sharp dissection are often necessary to adequately identify the ureter and its course. If the ureteral injury is discovered after the initial gynecologic procedure, the urologist must decide whether to enter through the original incision and approach the ureter transperitoneal or to make a new incision and approach the ureter using a retroperitoneal approach. Either approach is acceptable, and each has distinct advantages and disadvantages. If one decides to enter through a previous midline incision, intraperitoneal adhesions may complicate the dissection; however, this approach spares the patient an additional incision.
In contrast, if a modified Gibson incision is made to approach the ureter retroperitoneally, the dissection may be less challenging technically because it avoids the adhesions of the peritoneal cavity, but the patient is left with an additional incision. Regardless of the approach, a Foley catheter is placed and the patient is prepared and draped in a sterile manner. In the transperitoneal approach, an incision is made through the scar of the old incision. The dissection is extended down to the peritoneal cavity, and once the small bowel and colon are identified, a vertical incision is made along the left side of the small bowel mesentery. Blunt dissection is performed in the retroperitoneum until the desired ureter is identified. If the inferior mesenteric artery limits the exposure, it can be divided without consequence. If the left lower ureter is the area of the injury, the sigmoid can be mobilized medially to gain adequate exposure.
In the retroperitoneal approach, after the incision is made, the external oblique, internal oblique, and transverses abdominus muscles are dissected in a muscle-splitting manner. Once the transversalis fascia is incised, take care not to enter the peritoneal cavity. The peritoneum and its contents are retracted medially, and the ureter is located in its extraperitoneal position. The ureter is most consistently found at the bifurcation of the common iliac artery, but it is often difficult to identify, especially when dilated. Steps that can differentiate the ureter from a blood vessel with a similar appearance include pinching the structure with forceps and watching for peristalsis. If peristalsis occurs, the ureter has been identified. Additionally, a fine needle can be placed into the lumen of the questionable structure. If urine is retrieved through aspiration, the ureter has been identified; if blood is aspirated, the structure is a blood vessel. Once the ureter is identified and dissected from its surrounding tissues, the diseased segment is excised. Take particular care not to disrupt the adventitia of the ureter, because its blood supply is contained within this layer. If the difficulty is encountered in identifying the diseased segment, retrograde ureteropyelography can be performed to aid in localizing the lesion. Another option is to place a ureteral catheter cystoscopically up to the lesion; the ureteral catheter can then be palpated during the ureteral dissection. Stay sutures are placed at each end of the ureter, and the ureter is mobilized enough so that tension-free anastomosis can be performed. Simple ureteroureterostomy is typically performed for ureteral lesions shorter than 2 cm. If the lesion is longer than 2 cm, or if it appears that the ureteral ends will not come together without tension, seek an alternative surgical approach. Options include further mobilization of the ureter, mobilization of the ipsilateral kidney, transureteroureterostomy, ureteroneocystostomy, ileal ureter interposition, or a combination of the above.
Once the ureter appears to have enough length to be anastomosed without tension, both ureteral ends are spatulated. Two 5-0 absorbable sutures are placed in through the apex of the spatulated side of one ureter and out through the nonspatulated side of the opposite ureter. Each suture is tied, and a running stitch is performed on one half of the ureter. The same steps are performed to complete the anastomosis on the opposite half of the anastomosis. Before completion of the second half, a double-J ureteral stent is placed by first placing a 0.038 cm Teflon-coated guidewire caudally and passing a standard 7F double-J stent over the wire. The wire is pulled after the position of the distal portion of the stent is confirmed within the bladder. Next, a small hole is made within the stent, such that the wire can be passed cephalad, placed into the proximal tip of the stent, and comes out of the created hole in the side of the stent. Once the position of the cephalad tip in the renal pelvis is confirmed, the wire is pulled, leaving a well-positioned stent.
After the anastomosis is completed, a Penrose drain or a Jackson-Pratt (JP) drain is placed in the retroperitoneum and is brought out through the skin. Omentum may be retrieved from a small incision in the posterior peritoneum and can be used to wrap the repair. Adjacent retroperitoneal fat may be used. The anterior abdominal fascia and skin are closed.
Transureteroureterostomy
Transureteroureterostomy (TUU) is a urinary reconstruction technique that is used to join one ureter to the other across the midline. It offers patients with distal ureteral obstruction an option to live without external urostomy appliances or internal urinary stents. TUU is also used in undiversion procedures when the surgeon wants to avoid the pelvis because of previous trauma, surgery, or radiation therapy. A transureteroureterostomy is approached best via a midline incision and can be performed using both intraperitoneal and extraperitoneal approaches. A left-to-right intraperitoneal transureteroureterostomy is described.
After a Foley catheter is placed and the patient is prepared and draped in a sterile manner, a midline incision is made, and the peritoneal cavity is opened. The small bowel is packed medially, and the posterior peritoneum lateral to the sigmoid and descending colon is incised to expose the ureter. The ureter is dissected, preserving its adventitia. The diseased portion of the ureter is identified, and a clamp is placed on the ureter proximal to the diseased portion. The diseased portion of ureter is excised, a stay stitch is placed on the proximal segment of the ureter, and the distal stump is ligated. The proximal ureter is dissected for a length of approximately 9 to 12 cm, while the adventitial vessels are preserved.
Attention is then turned to exposing the right ureter. The ascending colon is retracted medially while an incision is made through the posterior peritoneum lateral to the colon. Blunt dissection aids in the identification of the ureter. Approximately 4 to 6 cm above the level of transection of the left ureter, the right ureter is exposed to make room for an anastomosis.
A retroperitoneal tunnel is created via blunt dissection, and the left ureter is pulled through the tunnel by the stay suture. When the left ureter is pulled through, taking care not to wedge the ureter between the inferior mesenteric artery (IMA) and the aorta is important, because obstruction may result. Instead, the ureter should be passed either over or under the IMA and should not be angulated or be under any tension. If the ureter is too short and a tension-free anastomosis can only be performed with the ureter firmly wedged between the IMA and the aorta, it is appropriate to consider ligation of the IMA. If this maneuver is not performed and the ureter is left firmly between the IMA and the aorta, a fibrous reaction of the ureter typically occurs, which causes an obstruction that must be treated later with a surgical procedure.
The tip of the left ureter is spatulated, and the medial wall of the right ureter is incised using a hook blade for a distance just longer than the diameter of the lumen of the left ureter. Using 4-0 or 5-0 absorbable suture material, a suture is placed at each end of the ureteral incision from the outside in. Each stitch is run over the course of one half of the anastomosis. Before finishing the second side of the anastomosis, a stent is placed along the entire right ureter using the technique described in ureteral stent placement. The 2 stitches are tied to each other.
After the anastomosis is completed, a Penrose drain or a JP drain is placed in the retroperitoneum and is brought out through the skin. Omentum or any adjacent retroperitoneal fat may be used to wrap the repair. The anterior abdominal fascia and skin are closed.
Psoas Hitch
The psoas hitch ureteral reimplantation technique has been used with great success to bridge defects in ureteral length due to injury or planned resection. Several surgical principles have been historically stressed when performing this procedure, including adequate mobilization of the bladder, fixation of the bladder to the psoas tendon before reimplantation, the use of a submucosal nonrefluxing-type ureteral anastomosis, and a 6-week delay before attempting repair after a surgical injury. After a Foley catheter is placed and the patient is prepared and draped in a sterile manner, various incisions are acceptable, including a midline, a Pfannenstiel, or a suprapubic V-shaped incision. A midline incision is preferred if the patient has a pre-existing midline scar from a previous gynecologic operation. If entering the peritoneal cavity can be avoided, this incision is preferred. The peritoneal reflection is dissected off the bladder. Some advocate saline installation in the subperitoneal connective tissue as a way of facilitating this portion of the dissection. If a peritoneal defect is encountered, it can be closed with a running chromic suture. Once the peritoneum is dissected off the bladder, the peritoneum can be reflected medially. Attention is then turned to dissection and excision of the diseased ureteral segment. The diseased portion of the ureter is identified, and a clamp is placed on the ureter proximal to it. A diseased portion of ureter is excised, a stay stitch is placed on the proximal segment of the ureter, and the distal stump is ligated. The superior pedicle of the bladder is ligated on the ipsilateral side, and the bladder wall is incised transversely, a little more than halfway around the bladder, in an oblique manner across the middle of its anterior wall at the level of its maximum diameter. When this horizontal incision is closed vertically, the effect of the incision is the elongation of the anterior wall of the bladder so that the apex of the bladder can be positioned and fixed above the iliac vessels.
After the bladder incision is made, 2 fingers are placed into the bladder to elevate it to the level of the proximal end of the ureter. If the bladder does not reach the proximal ureter, several steps can be performed for additional length. These steps include extending the bladder wall incision laterally to obtain further length, or the peritoneum and connective tissue from the pelvic and lateral walls may be dissected from the contralateral side of the bladder. This dissection may require ligation and division of the superior vesical pedicle on the contralateral side. Once adequate mobilization of the bladder has occurred, the bladder is held against the tendinous portion of the psoas minor muscle without tension. Prolene sutures (2-0) are sutured into the bladder wall and to the tendon to fix the bladder in place.
With the bladder open, attention is turned to the ureteral reimplant. An incision is made in the bladder mucosa at the proposed site of the new ureteral orifice. A submucosal dissection occurs approximately 3 cm from the incision site so that a tunnel is created. Lahey scissors may be used to facilitate this dissection. After achieving a 3 cm tunnel length, the scissors are inverted and the tips are pushed through the bladder wall. An 8F feeding tube is passed over the scissor blades, and the stay suture on the proximal tip of the ureter is tied to the other end of the catheter so that traction on the catheter draws the ureter into the bladder. The ureteral tip is trimmed obliquely, and 4-6 absorbable sutures (4-0) are used to fix the ureter to the bladder mucosa. The ureteral adventitia is tacked to the extravesical bladder wall with several 4-0 absorbable sutures. A double-J ureteral stent may be placed at this time.
A non-tunneled reimplant is also an acceptable choice in most adults if the ureteral length is insufficient. The end of the ureter can be reflected back after making a small longitudinal incision from the tip proximally about 1.5 cm. This will make the end of the ureter into a nonrefluxing nipple, which is useful when there is an inadequate length for an antirefluxing submucosal tunnel.
After completing the reimplant, 2 fingers are placed within the bladder, while 5 or 6 absorbable sutures (2-0) are placed within the bladder muscle, the psoas muscle, and the psoas minor tendon, paying specific attention not to suture the genitofemoral nerve.
Alternatively, sutures may also take deep bites in the muscle itself. The bladder is closed with a 3-0 running absorbable suture on the mucosa and a running 2-0 suture incorporating the bladder muscle and adventitial layers. A Penrose drain or a JP drain is placed in the retroperitoneum next to the bladder closure. The anterior abdominal fascia and the skin then are closed.
Boari Flap
A Boari flap may be required to bridge long defects of the middle and lower ureter to the bladder. Laparoscopic construction of a Boari flap was performed in a patient with a ureteral stricture secondary to iatrogenic injury. The salient steps performed were spatulation of the transected ureteral end, fashioning of a Boari flap from the bladder, end-to-side anastomosis of the ureter to the flap, placement of a stent with the aid of a suction cannula, and closure of the flap over the stent. A Boari flap can be accomplished laparoscopically with minimal morbidity.
After preparing and draping the patient, a midline or Pfannenstiel incision is made. Once the transversalis fascia is incised, the ureter may be approached either transperitoneal or retroperitoneally. In the transperitoneal approach, the peritoneal cavity is entered, the sigmoid or cecum is reflected medially, the posterior peritoneum is incised, and the ureter is identified. In the retroperitoneal approach, care is taken not to enter the peritoneal cavity, the peritoneum is mobilized medially, and the ureter is identified and exposed. A stay stitch is placed in healthy ureter tissue just proximal to the injury. The remaining end of the ureter is tied off.
The peritoneum is then dissected from the wall of the bladder. This dissection may be facilitated with hydro dissection, in which saline is injected subperitoneally, separating the peritoneal layer from the muscle layers of the bladder. The necessary length of the bladder flap (i.e. the distance between the posterior wall of the bladder and the end of the healthy proximal ureter) is measured with an umbilical tape, the bladder is one-half full of saline, and the length and shape of the bladder flap are planned. To measure accurately on the dome of the bladder, several stay stitches are placed at the base of the proposed bladder flap and at the apex. The bladder flap should be planned with a large base because the base will contain the blood supply for the flap. The length of the bladder flap (i.e. the distance between the base and apex) should equal the distance between the posterior wall of the bladder and the end of the healthy proximal ureter. The width of the apex should be at least 3 times the diameter of the ureter to prevent constriction after the flap is tubularized. Avoid scarred areas of the bladder.
After proper planning, an outline of the flap is made in the bladder wall with coagulating current, and the bladder flap is remeasured. If the measurements are satisfactory, the bladder flap is cut via cutting current, and the concomitant bleeding vessels are coagulated.
After the bladder flap is turned superiorly, Lahey scissors are used to prepare a ureteral tunnel. The tunnel should be at least 3 cm long and is created by placing the Lahey scissors submucosally at the apex of the flap, tunneling the appropriate distance, and coming out through the mucosa. Submucosal injection of saline may aid in this dissection. An 8F feeding tube is pulled through the tunnel by the scissors and the stay suture on the proximal ureter is tied to the feeding tube after the ureteral end is spatulated. The feeding tube is pulled toward the bladder, followed by the ureter. The stay suture is cut after the ureter has traveled completely through the tunnel.
The bladder flap is sutured to the psoas tendon of the psoas minor with a few 2-0 absorbable sutures. These sutures fix the flap in place to prevent tension on the ureteral anastomosis. The ureter is anastomosed to the bladder mucosa with several 4-0 absorbable sutures. A few of the sutures should include the muscle layer of the bladder to fix the ureter into place. An 8F feeding tube is passed up the ureter into the renal pelvis and out through the bladder and body wall.
Before closing the bladder, a large suprapubic tube is placed, i.e. either a 22-24F Malecot or Foley. Then, the bladder is closed by approximating the bladder mucosa with a 3-0 absorbable running suture followed by a second row of running sutures, which approximates the muscularis and adventitial layers. A few absorbable sutures (5-0) can be placed to approximate the distal end of the flap to the adventitia of the ureter. If a transperitoneal approach is used, close the peritoneum and then place a Penrose or a JP drain retroperitoneally adjacent to the bladder closure. The anterior abdominal fascia and skin are closed.
Postoperative Details
Ureteral Stent
After the patient has recovered from anesthesia and is in suitable condition, the patient may be discharged with instructions to return to the clinic in 14 to 21 days, when the stent will be removed. The patient is discharged with 3 days of antibiotics and oral analgesics for potential bouts of discomfort from the stent.
Ureteroureterostomy, Transureteroureterostomy, Psoas Hitch and Boari Flap
Patients who underwent a transperitoneal approach are kept on a regimen of nothing by mouth (NPO) for the first day after surgery. Subsequently, signs of bowel function are monitored routinely. Once bowel sounds are present, the diet is advanced to clear liquids, and when the patient passes flatus, a regular diet is instituted. Patients who undergo a retroperitoneal approach are started on clear liquids on the first day after surgery unless they are nauseous. Their diets are also advanced when they have passed flatus. All patients receive a patient-controlled anesthetic (PCA) pump postoperatively unless they had an epidural catheter placed intraoperatively. They are then given an epidural pump. Oral analgesics are administered after patients tolerate a regular diet. All patients receive a 24 hours course of intravenous antibiotics to prevent wound infections. Patients are encouraged to ambulate on the first day after surgery. Once the pain is controlled with oral analgesics and patients are tolerating a regular diet, they are eligible for discharge, with or without their drains. If drains are not removed in the hospital, set appointments to assess patients and their drains in the clinic.
Follow-up
In patients who do not require a cystotomy, the Foley catheter or suprapubic tube is left to drain the bladder until the drain output from the Penrose or JP drain is less than 30 ml per day. If this is achieved, the Foley catheter can be removed or the suprapubic tube can be clamped, and the output from the Penrose or JP drain is monitored. If no drainage occurs, the drain can be removed. If drainage increases from the previous level, the Foley catheter is replaced, or the suprapubic tube is unclamped. After several days, the same sequence of events occurs to determine whether the ureter has healed completely. If a stent or feeding tube is used, it can be removed 7 to 10 days after surgery.
In patients who require cystotomy, the Foley catheter or suprapubic tube is left in place for 7 to 10 days after surgery, at which time cystography is usually performed. If no extravasation is observed during the cystogram, the Foley catheter or suprapubic tube can be removed. At the same time, the outputs from the Penrose or JP drain are monitored. If no drainage occurs, the drain can be removed. If drain output increases from the previous level, the Foley catheter is replaced. After several days, the same sequence of events occurs to determine whether the ureter has healed completely. If a stent is used, the stent is removed 10 to 14 days after surgery.
Laparoscopic surgery has become a surgical discipline in its own right. Like any other surgical technique, it does involve a risk of complications. This risk of ureteral injury is related to the complexity of the laparoscopic procedure. The set-up phase for laparoscopy must never be considered banal. The methods of postoperative monitoring must be adapted to take into account the shorter hospital stay and the fact that a considerable number of ureteral injuries go unnoticed intraoperatively.
Conclusion
• Awareness of risk factors and good experience in laparoscopy are the main factors in preventing and reducing ureteric injuries.
• Immediate and early diagnosis of ureteral injuries gives excellent outcome and minimal morbidity. While delayed diagnosis had prolonged morbidity.
• For any suspicion of ureteric injury investigation should be done to role out the injury.
• Placement of stent is helpful especially in difficult cases reduce the rate of ureteric injury.
• Intraoperative cystoscopy during laparoscopy leads to an immediate diagnosis of ureteric injuries.
• Excessive use of diathermy near the ureter follow by thermal injury.





