Robotic Cholecystectomy
Introduction
In general surgery, advanced robotics will likely find its place in the most complex laparoscopic procedures where the enhanced dexterity and superior visualization will extend the feasibility of the minimally invasive approach, particularly in patients requiring advanced suturing and precise tissue dissection. Robot-assisted laparoscopic cholecystectomy is a safe and effective bridge to advanced robotics in general surgery.
Laparoscopic cholecystectomy is a prime operation with which to begin robot applications for several reasons. First, gallstone disease is the most common of all abdominal diseases for which patients undergo a laparoscopic procedure. Moreover, it is an operation with a very standardized approach to prevent complications. This standardized approach has an added advantage of having aspects that may be more broadly applied to other more complex minimally invasive operations. For example, the dissection of the Calot triangle is analogous to dissection and isolation of vasculature, the cystic duct and artery can be tied instead of using clips, and removal of the gallbladder from the gallbladder fossa requires avascular dissection and the appreciation of tissue planes. Therefore, robotic cholecystectomy may allow general surgeons to acquire clinical da Vinci experience in a familiar setting.
Segments of Robotic Cholecystectomy
Segment Operative Tasks:
• Skin incision, port placement, exploration, adhesiolysis, patient positioning, and robotic arm draping
• Positioning of da Vinci, docking of robot arms and camera
• Initial dissection of gallbladder until placement into the endoscopic bag
• Gallbladder extraction, the performance of additional procedures, and incision closure.
Preoperative Preparation
Indications and preoperative preparation is similar to conventional laparoscopic procedure. An informed consent is to be taken from the patient explaining the new technology and its possible complications. A prophylactic dose of intravenous antibiotic is given just prior to the commencement of surgery.
An orogastric or nasogastric tube is placed prior to the creation of pneumoperitoneum to decompress the stomach. Sequential compression devices placed on legs for DVT prophylaxis. The footboard is placed to keep the patient from sliding off the table. A seatbelt is tied in the midthigh region.
Patient Position
The patient is placed in a supine position and the right arm is tucked by the side of the patient. Standard surgical preparation is done from the nipple line to the thigh. A standard protection bar over the patient’s head may easily block the robotic arms, giving protection to the head. Convex protection shields or bars that protect the patient’s nose and the ventilation tube are good protection for robotic-assisted surgical interventions.
Pneumoperitoneum is created with a closed (veress needle) or open technique. 12 mm camera port is inserted at the umbilicus which should be around 20 cm away from the target anatomy (cystic pedicle). Diagnostic laparoscopy is done. Then, a mild left tilt of the table is done with reverse Trendelenburg position.
Port Position
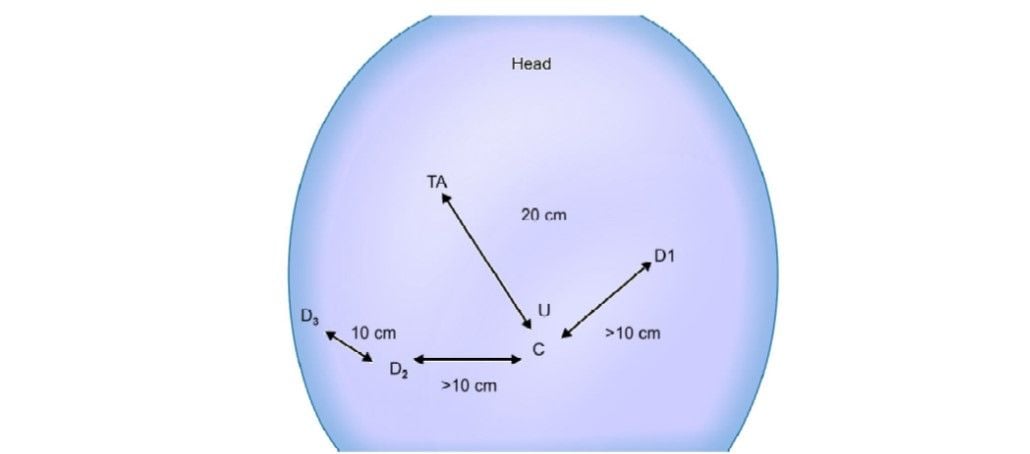
Three 8 mm da Vinci ports are inserted at least 10 cm apart for the robotic arm placement. The remote center of the ports is placed at the level of the abdominal wall.
Robotic arm 1: Left midclavicular line, below the subcostal margin.
Robotic arm 2: Right midclavicular line, 5 to 10 cm below the subcostal margin.
Robotic arm 3: 10 cm below and lateral to robotic arm 2. (Optional, some surgeons use a 5/12 mm assistant port rather than a robotic arm as the third port)
Docking and Operating Room Set-up
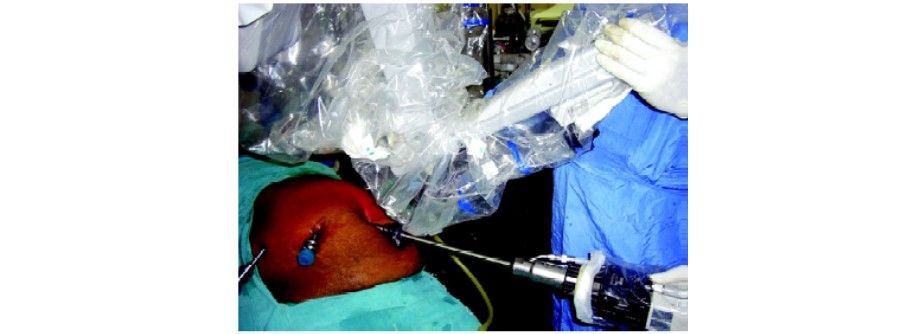
The arms are draped with disposable sterile drapes. The patient's cart is brought over the patient’s right shoulder. This implies that the robot is located behind the operating field and that the robotic arms cross this area and seem to work in a backward direction. The center column of the robotic cart acts as the vertical central axis of the robot. This column should be positioned at the far end of an axis running from the umbilicus through the hilar region of the liver.
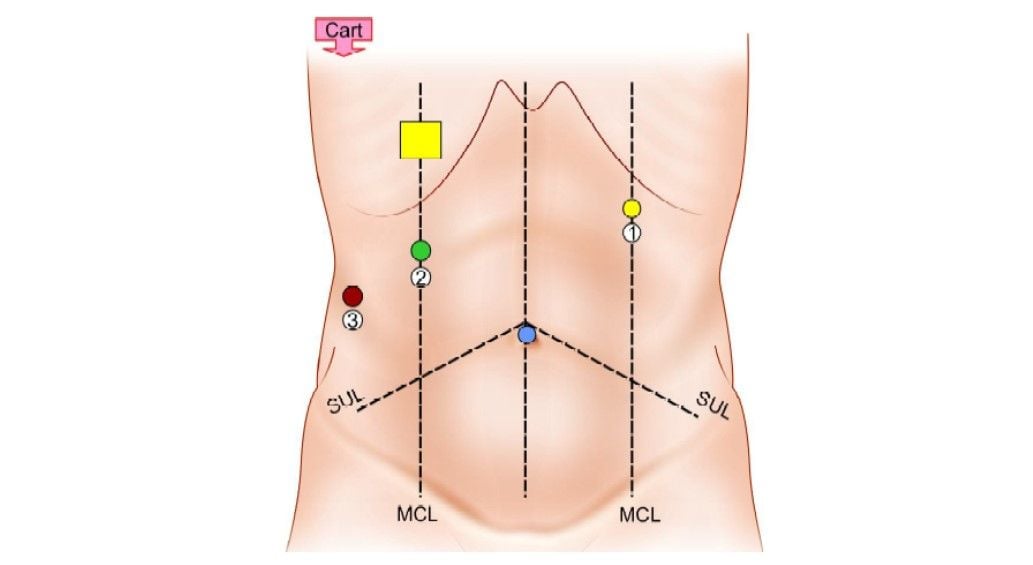
The patient table should be tilted according to the surgeon’s choice before docking. Align the “sweet spot” for proper camera arm positioning. Align the clutch button, third set-up joint, and the center column. Allow 45° angle between each arm. Dock the camera arm and other arms to the respective ports. The camera and the instruments are placed in the ports.
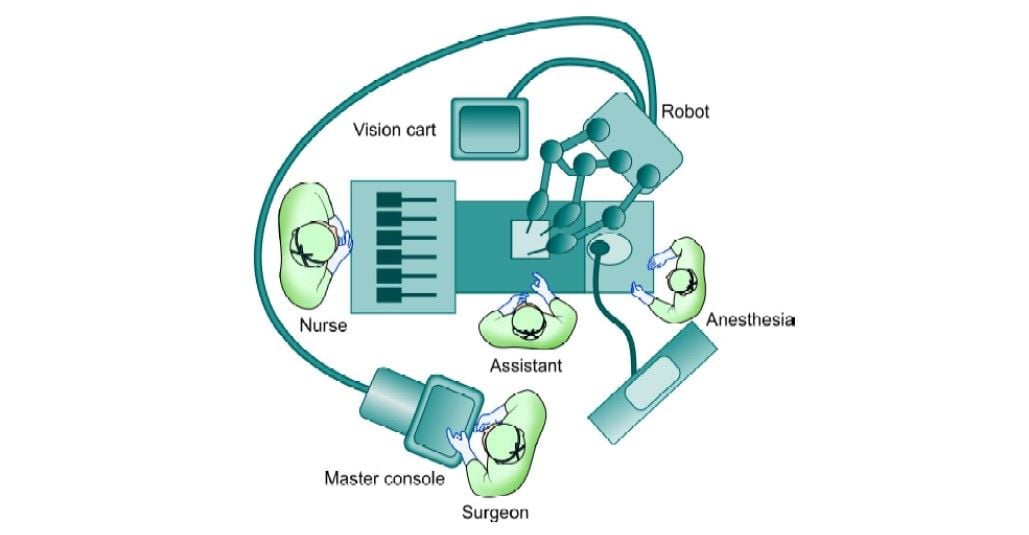
The operating room set-up is shown in the line diagram below.
Step 1 (Diagnostic Laparoscopy and Port Position)
The patient is kept in a supine position. Parts cleaned and draped from nipple line to midthigh. Pneumoperitoneum is created with veress needle at an umbilical position. A 12 mm camera port is inserted at the umbilicus using optiview cannula. Diagnostic laparoscopy is done and the gallbladder visualized. The patient is positioned in a reverse Trendelenburg position with a left tilt. Three da Vinci 8 mm ports are inserted under vision as per the guiding principles described above. Any adhesiolysis if required is done at this particular time with standard laparoscopic instruments.
Step 2 (Docking of Robotic Cart)
The patient cart is brought over the right shoulder of the patient. Docking of camera arm is done first followed by instrument arms. The principles of docking are kept in mind and a system check-up is done after docking is complete. Instruments are inserted under endoscopic vision R1: Maryland dissector R2 and R3: Prograsp forceps.
Step 3
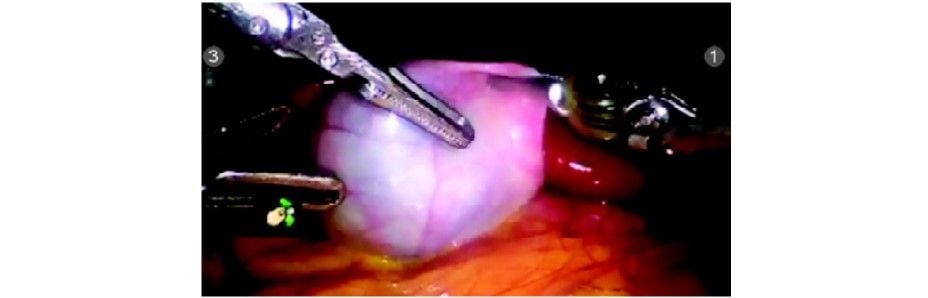
The fundus is grasped with pro grasp forceps (R3) and pushed towards the right shoulder of the patient. This traction is constant once the arm is locked unlike in laparoscopy where it depends on the expertise of the holding assistant. Care should be taken to avoid injury to the diaphragm because of the inadvertent slippage of the fundus from the grasper. Due to the lack of haptic feedback to the main surgeon, it can go unnoticed and cause trauma to the diaphragm.
Step 4
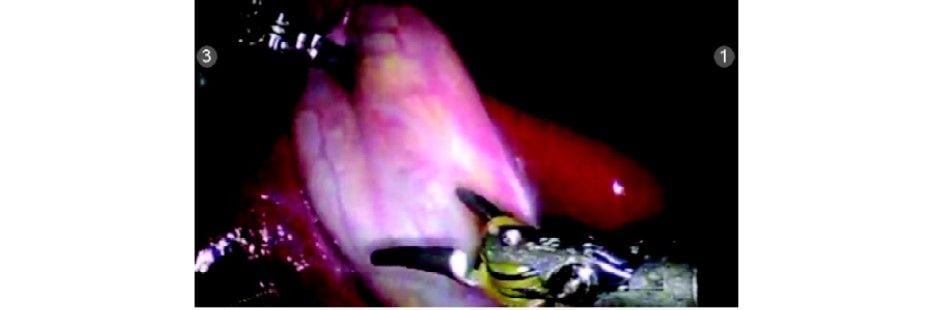
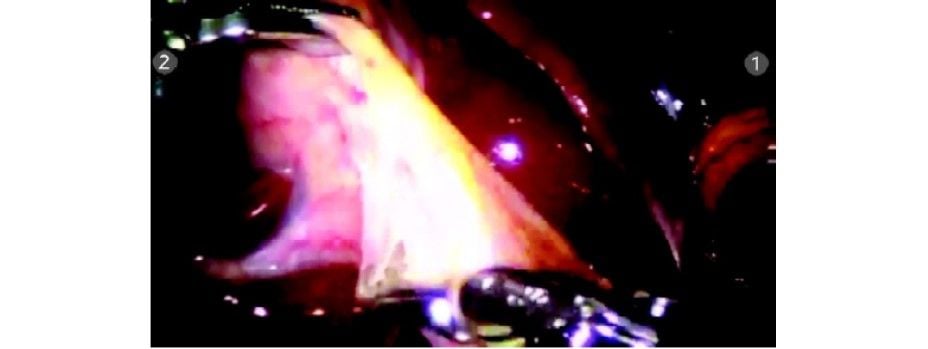
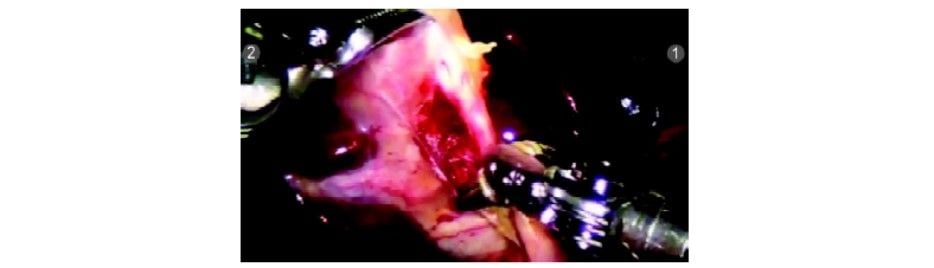
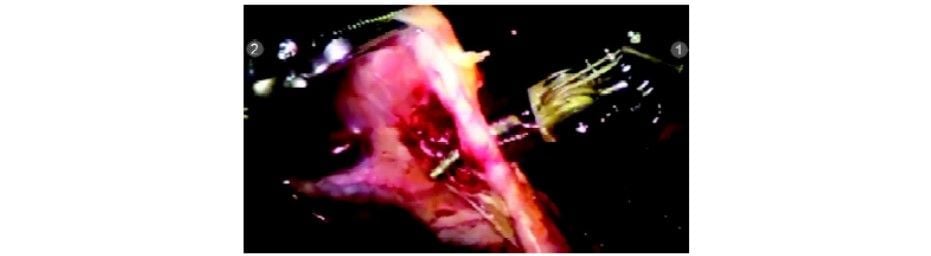
Hartmann’s is grasped with pro grasp forceps (R2) and Calot’s triangle dissected with Maryland Endowrist instruments (R1). Seven degrees of freedom in the Endowrist instruments gives a distinct advantage in this dissection.
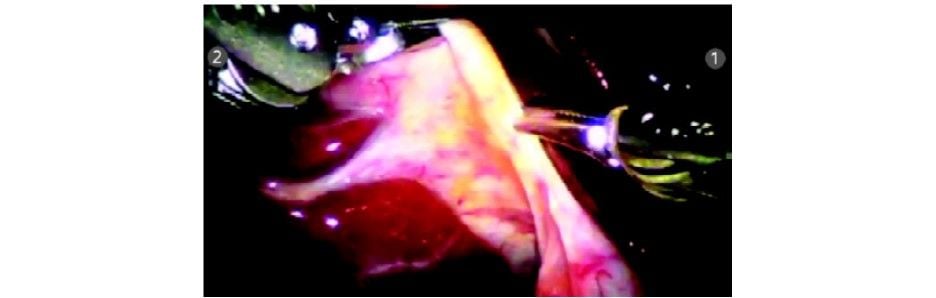
Step 5
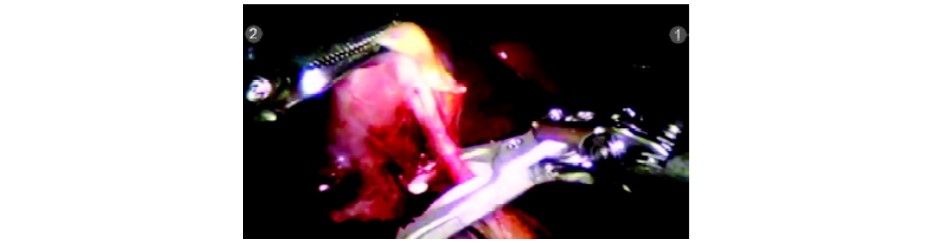
The cystic duct and artery are dissected completely. Clips are applied with Endowrist hemolock clip applicator (R1).
The cystic duct and artery are divided with the Endowrist scissors (R1) in between the clips. The Endowrist movements enable the surgeon to cut precisely in the direction required.

The gallbladder is dissected off the gallbladder (GB) fossa with the help of a hook electrode with monopolar cautery (R1). The Endowrist function of the hook provides a lot of versatile movements that aid in this step of surgery.

Just before taking off the gallbladder from the fossa, it is retracted cephalad and the GB fossa is inspected for any bleed or bile leak.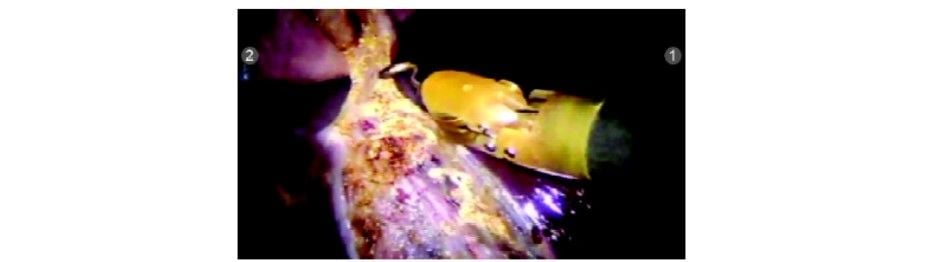 Inspection of GB fossa
Inspection of GB fossa
Step 9
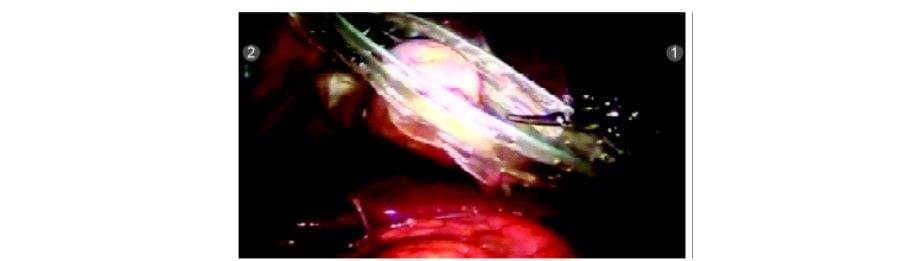
The R3 arm is undocked and endobag is inserted by the patient side assistant surgeon using a standard laparoscopic grasper. Any suction/irrigation can also be done by the assistant surgeon by undocking R3. The gallbladder is placed in the endobag and extracted out.
Step 10
Check for hemostasis and clip position. Remove instruments and ports under vision. Incisions are closed in layers.
Discussion
Laparoscopic cholecystectomy for the treatment of biliary colic is a relatively straightforward, routine procedure. It will be difficult to defend the use of a highly developed and expensive surgical tool that requires additional time for preparation and larger incisions for the trocars, especially while the advantages of such tools are hard to prove for this specific procedure.
There are two situations in which robotics can be applied to this particular procedure:
1. Complicated gallstone disease, like acute cholecystitis, CBD exploration or choledochoenterostomy.
2. Ideal learning and teaching environment for gastrointestinal robot-assisted surgery.
Advantages
Robotic instruments clearly provide superiority in some aspects of the surgery. The increased degrees of freedom (wrist action) allow the surgeon to easily reach behind structures and negotiate difficult surgical angles. The ergonomics of the operating surgeon can be significantly improved by operating in the sitting position and facing directly forward toward the 3D operative image. These can be of value in cases of acute and chronic cholecystitis, where vision is impaired by inflammation, where there is an increased tendency for diffuse bleeding in the dissection field, and edema and fibrosis make the dissection difficult.
By adjusting movement scaling, surgeon tremors can be significantly reduced; thereby, increasing operative precision. The translation of the surgeon’s hand and wrist movements are reliable and can be scaled down to improve precision and increase steadiness.
Limitations
There is a need for more variety in the type and size of robotic trocars. The robotic arms are bulky, taking up significant space and limiting the capability of the surgical assistant. Furthermore, the da Vinci system is not attached to the operating table, so the position of the table cannot be changed without undocking the instrument and camera. The robotic arms lack haptic feedback, so the surgeon must rely on visual cues to avoid stretching and damaging tissue or suture. Financially, surgical robotic systems remain quite expensive and are associated with significant operating costs. Current robotic systems and instrumentation are still considered to be first-generation and should improve as the technology evolves.
Conclusion
Robotic cholecystectomy is feasible and safe. There is a significant learning curve (around 20–30 cases) to gain experience for setting-up the robotic instrumentation, which appears to be much less steep for the actual use of the machine.
It is unlikely that robotic cholecystectomy will be routinely performed in the near future. Further studies are needed to identify the benefits to the patient and compare it to the additional cost of robotic cholecystectomy before routine application of this technique can be justified. However, robotic cholecystectomy may prove its value in cases of complex gallstone disease and is an excellent procedure for teaching the basics of robotic surgery.
Robotic Coronary Bypass Surgery
Four ports are used and a robotic arm is introduced. One of the arms contains a telescope which sends 12 to 15 times a magnified clear image on the monitor. Another arm is for a stabilizer which is used to hold the diseased coronary artery in place while bypass is performed. The other two remaining right and the left instrument is used to perform the microvascular anastomosis. Currently, robotic coronary bypass surgery should be considered only for the patient who has single-vessel disease but in near future, we have hope to perform double or even triple bypass surgery.
The attached instruments are controlled by the surgeon, who sits at an adjacent console. Several passive mechanical devices, primarily used to hold the telescope, have been developed as assistants for general laparoscopic surgery. They successfully reduce the stress on the surgeon by eliminating the inadvertent movements of a human assistant, which can be distracting and disorienting.
More and more surgeries from the prostate to the heart are being performed by doctors remotely guiding robotic arms. In such procedures, the surgeon’s hands never enter the patient. After the initial incisions are made, robotic arms wielding a tiny camera and surgical tools make the snips, stanch the blood flow, and sew up inside when all is done. The surgeon sits at a console usually in the operating room, although the technology would allow a doctor to operate on a patient on the other side of the world peering into binocular-like lenses at views provided by the camera inside the patient. The doctor guides the robot’s work by twisting his wrists in the stirrup- like handles, moving his thumb and forefinger in scissor-like loops, or tapping foot pedals to focus the camera or move a robotic arm.
Robotic Prostatectomy
The most common robotic surgery is radical prostatectomy. Its use has grown from just 1,500 procedures in 2000 to an estimated 20,000 last year. More than 8,000 prostate glands were removed robotically last year, up from 36 in 2000. The procedure accounted for more than 10 percent of the 75,000 prostatectomies done in 2004. Busy professionals like the fact that they can be out of the hospital in a day, vs. two or three for open surgery, and resume normal activities in one week rather than six. Cutting nerves around the prostate can lead to incontinence or impotence, so precision is important. Between 25 and 60 percent of conventional prostatectomy patients suffer from postoperative impotence. Small studies of robotic surgery have shown at least a short-term benefit in terms of impotence and incontinence.
Not every patient is a candidate. Complicated cases when the patient is very sick, need multiple procedures, or has had previous chest surgery are not suited to robotics. Nor can a heart transplant or artificial heart implant be done this way. If the complication arises, the patient may wind-up being opened. So patients who want robotics smaller scars and quicker recovery times will need to ask hard questions. Find out how many times, the procedure you need has been done robotically and what the advantages and disadvantages are. Just as important, ask how many robotic surgeries, the doctor who will do yours has performed. In real life, you want the force of experience to be with you.
For qualified candidates, the robotic prostatectomy offers numerous potential benefits over the traditional open prostatectomy, including:
• Shorter hospital stay
• Less pain
• Less risk of infection
• Less blood loss and transfusions
• Less scarring
• Faster recovery
• Quicker return to normal activities.
While still a technology that is in its infancy, the use of robots to assist in surgery is becoming more and more widespread. There are now large case series reported in the literature that show possible benefits to the patient in terms of recovery from such surgeries as prostate and cardiac procedures. Additionally, as these systems continue to develop, improved technology and software provide the surgeon with “assistance” that improves precision and accuracy.
Robotic technology requires a tremendous financial investment, so you might not see it at every community hospital in the near future. However, as with all technology, the price will likely fall quickly as the applications are expanded, as more widespread adoption occurs, and as the field of robotics experiences additional breakthroughs. For now, many major academic institutions are beginning to purchase and deploy these systems.
Technology allows for amazing things to be accomplished. Robotic surgery is a new and expensive tool that is beginning to see adoption. Minimally invasive surgery with a robot and without a bypass is the next logical step in the development of cardiac surgery. It is beginning to show clear benefits for patients and this means that it will likely become more and more popular with time and as the price falls.
Robotic Versus Laparoscopic Prostatectomy
In laparoscopy, the surgeon uses long instruments through small openings and maneuvers them with direct hand contact. Robotic systems use even more delicate instruments that possess two additional degrees of movement excursion (for a total of six, as with a human hand). Comfortably seated at the robot console, the surgeon can maneuver the instruments via a computer interface.
Laparoscopic radical prostatectomy is associated with a steep learning curve. Even in the hands of expert surgeons, laparoscopic radical prostatectomy requires extensive learning; approximately 40 cases are needed to master this technique. In contrast, the learning of robotic prostatectomy seems to be more intuitive and less demanding.
Robotic prostatectomy is a safe, effective, and reproducible technique for removing the prostate. In most patients, it can be performed within one and a half to two hours with minimal blood loss and few complications. The procedure incorporates principles of both laparoscopic and open radical prostatectomy. The patients enjoy the benefits of surgical treatment in the setting of less invasion, minimal pain, limited blood loss, and early functional and overall recovery.
Introduction
In general surgery, advanced robotics will likely find its place in the most complex laparoscopic procedures where the enhanced dexterity and superior visualization will extend the feasibility of the minimally invasive approach, particularly in patients requiring advanced suturing and precise tissue dissection. Robot-assisted laparoscopic cholecystectomy is a safe and effective bridge to advanced robotics in general surgery.
Laparoscopic cholecystectomy is a prime operation with which to begin robot applications for several reasons. First, gallstone disease is the most common of all abdominal diseases for which patients undergo a laparoscopic procedure. Moreover, it is an operation with a very standardized approach to prevent complications. This standardized approach has an added advantage of having aspects that may be more broadly applied to other more complex minimally invasive operations. For example, the dissection of the Calot triangle is analogous to dissection and isolation of vasculature, the cystic duct and artery can be tied instead of using clips, and removal of the gallbladder from the gallbladder fossa requires avascular dissection and the appreciation of tissue planes. Therefore, robotic cholecystectomy may allow general surgeons to acquire clinical da Vinci experience in a familiar setting.
Segments of Robotic Cholecystectomy
Segment Operative Tasks:
• Skin incision, port placement, exploration, adhesiolysis, patient positioning, and robotic arm draping
• Positioning of da Vinci, docking of robot arms and camera
• Initial dissection of gallbladder until placement into the endoscopic bag
• Gallbladder extraction, the performance of additional procedures, and incision closure.
Preoperative Preparation
Indications and preoperative preparation is similar to conventional laparoscopic procedure. An informed consent is to be taken from the patient explaining the new technology and its possible complications. A prophylactic dose of intravenous antibiotic is given just prior to the commencement of surgery.
An orogastric or nasogastric tube is placed prior to the creation of pneumoperitoneum to decompress the stomach. Sequential compression devices placed on legs for DVT prophylaxis. The footboard is placed to keep the patient from sliding off the table. A seatbelt is tied in the midthigh region.
Patient Position
The patient is placed in a supine position and the right arm is tucked by the side of the patient. Standard surgical preparation is done from the nipple line to the thigh. A standard protection bar over the patient’s head may easily block the robotic arms, giving protection to the head. Convex protection shields or bars that protect the patient’s nose and the ventilation tube are good protection for robotic-assisted surgical interventions.
Pneumoperitoneum is created with a closed (veress needle) or open technique. 12 mm camera port is inserted at the umbilicus which should be around 20 cm away from the target anatomy (cystic pedicle). Diagnostic laparoscopy is done. Then, a mild left tilt of the table is done with reverse Trendelenburg position.
Port Position

Port positioning (TA: Target anatomy)
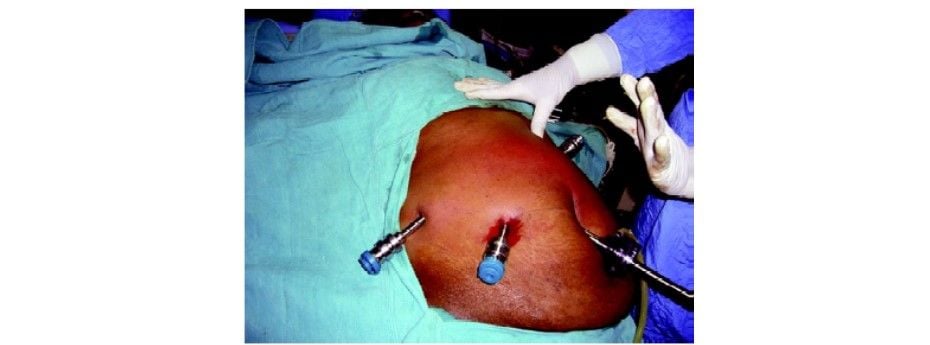
Three 8 mm da Vinci ports are inserted at least 10 cm apart for the robotic arm placement. The remote center of the ports is placed at the level of the abdominal wall.
Robotic arm 1: Left midclavicular line, below the subcostal margin.
Robotic arm 2: Right midclavicular line, 5 to 10 cm below the subcostal margin.
Robotic arm 3: 10 cm below and lateral to robotic arm 2. (Optional, some surgeons use a 5/12 mm assistant port rather than a robotic arm as the third port)
Docking and Operating Room Set-up
The arms are draped with disposable sterile drapes. The patient's cart is brought over the patient’s right shoulder. This implies that the robot is located behind the operating field and that the robotic arms cross this area and seem to work in a backward direction. The center column of the robotic cart acts as the vertical central axis of the robot. This column should be positioned at the far end of an axis running from the umbilicus through the hilar region of the liver.

Position of patient cart and ports
The patient table should be tilted according to the surgeon’s choice before docking. Align the “sweet spot” for proper camera arm positioning. Align the clutch button, third set-up joint, and the center column. Allow 45° angle between each arm. Dock the camera arm and other arms to the respective ports. The camera and the instruments are placed in the ports.
The operating room set-up is shown in the line diagram below.

Operating room set-up for cholecystectomy
Operative TechniqueStep 1 (Diagnostic Laparoscopy and Port Position)
The patient is kept in a supine position. Parts cleaned and draped from nipple line to midthigh. Pneumoperitoneum is created with veress needle at an umbilical position. A 12 mm camera port is inserted at the umbilicus using optiview cannula. Diagnostic laparoscopy is done and the gallbladder visualized. The patient is positioned in a reverse Trendelenburg position with a left tilt. Three da Vinci 8 mm ports are inserted under vision as per the guiding principles described above. Any adhesiolysis if required is done at this particular time with standard laparoscopic instruments.

Camera port insertion under vision with 0° endoscope and optiview cannula
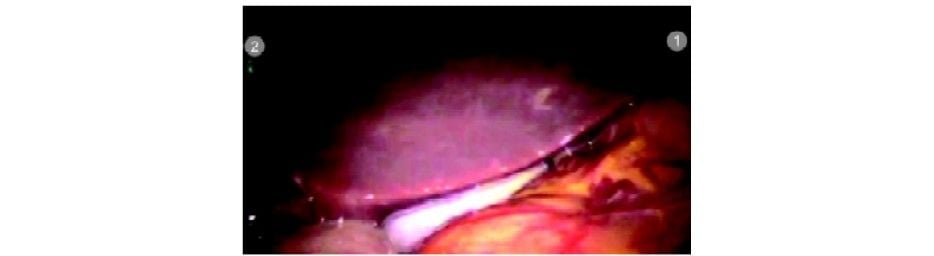

Diagnostic laparoscopy
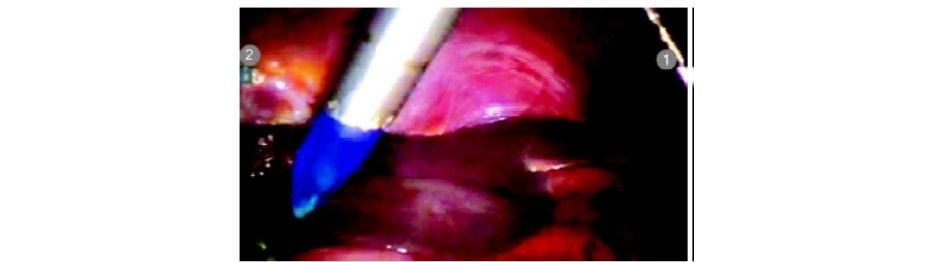
da Vinci port placement under the vision

da Vinci port placement under the vision

Final port position for robotic cholecystectomy
Step 2 (Docking of Robotic Cart)
The patient cart is brought over the right shoulder of the patient. Docking of camera arm is done first followed by instrument arms. The principles of docking are kept in mind and a system check-up is done after docking is complete. Instruments are inserted under endoscopic vision R1: Maryland dissector R2 and R3: Prograsp forceps.

Docking the camera arm
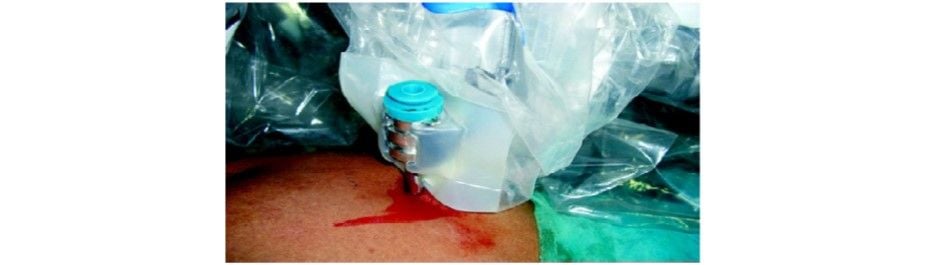

Docking the instrument cannula to the instrument arm
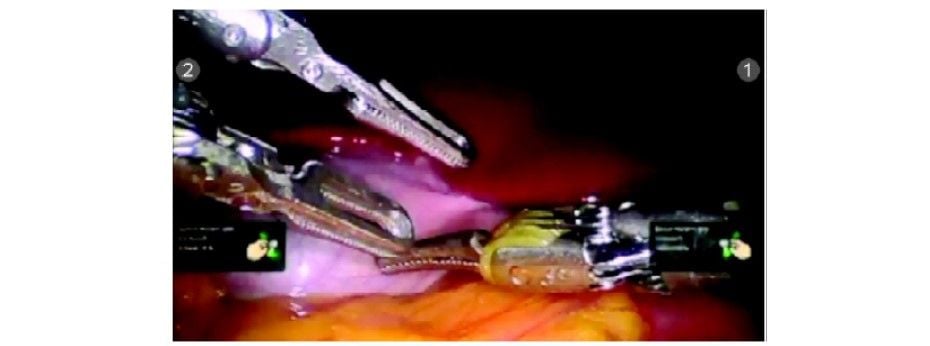

Insert the instruments under endoscopic vision
Step 3
The fundus is grasped with pro grasp forceps (R3) and pushed towards the right shoulder of the patient. This traction is constant once the arm is locked unlike in laparoscopy where it depends on the expertise of the holding assistant. Care should be taken to avoid injury to the diaphragm because of the inadvertent slippage of the fundus from the grasper. Due to the lack of haptic feedback to the main surgeon, it can go unnoticed and cause trauma to the diaphragm.

Holding the fundus with R1 Maryland dissector so as to enable R3 grasping forceps to retract it

Pushing the fundus towards the right shoulder
Step 4
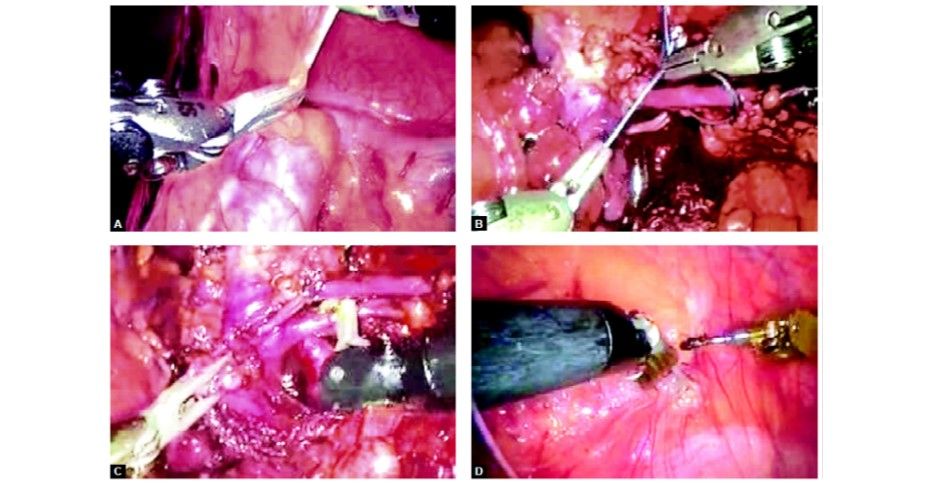
Hartmann’s is grasped with pro grasp forceps (R2) and Calot’s triangle dissected with Maryland Endowrist instruments (R1). Seven degrees of freedom in the Endowrist instruments gives a distinct advantage in this dissection.

Hartmann’s is grasped by R2 and Calot’s being dissected by Maryland in R1

Posterior peritoneum being dissected off the Calot’s triangle

Posterior window creation

Cystic duct being dissected using the degrees of freedom of the Maryland dissector
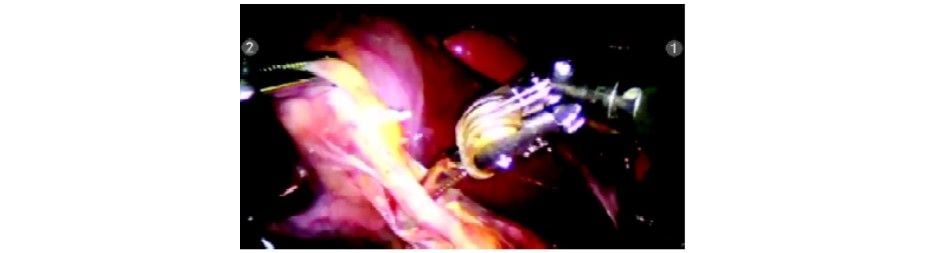
Window made posterior to the cystic duct

Window made posterior to the cystic duct
Step 5
The cystic duct and artery are dissected completely. Clips are applied with Endowrist hemolock clip applicator (R1).

The clip being applied by robotic clip applicator. The degrees of freedom can be used for optimum clip application
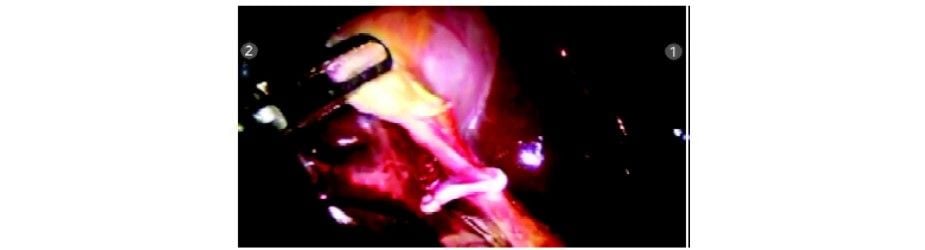
Clip applied to the cystic duct
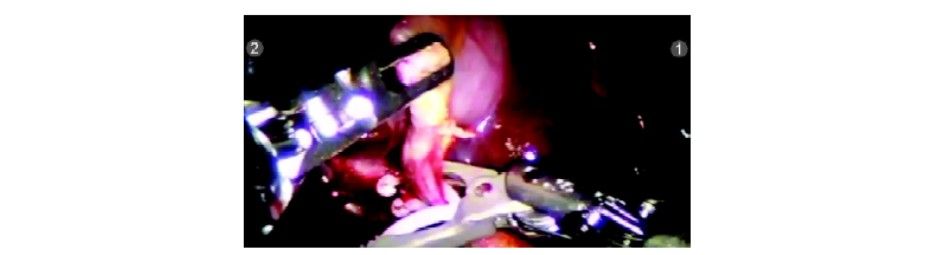

Clip applied to the cystic duct

The second clip being applied
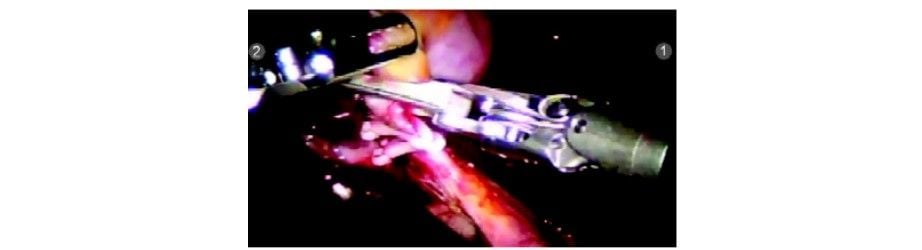
The third clip being applied
Step 6
The third clip being applied
The cystic duct and artery are divided with the Endowrist scissors (R1) in between the clips. The Endowrist movements enable the surgeon to cut precisely in the direction required.

Cystic duct being divided with scissors
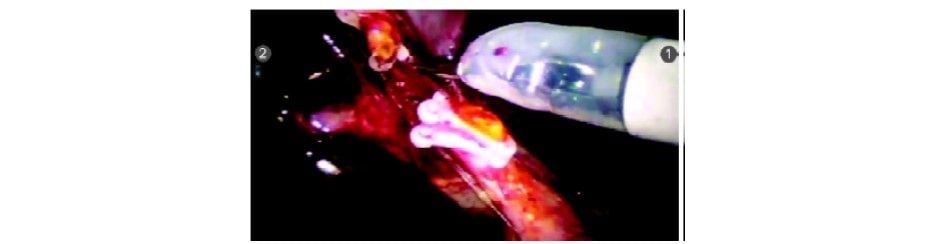
Cystic duct divided

Cystic artery dissected
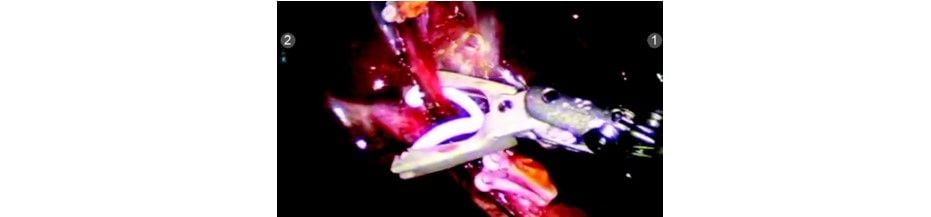
Clip applied to cystic artery
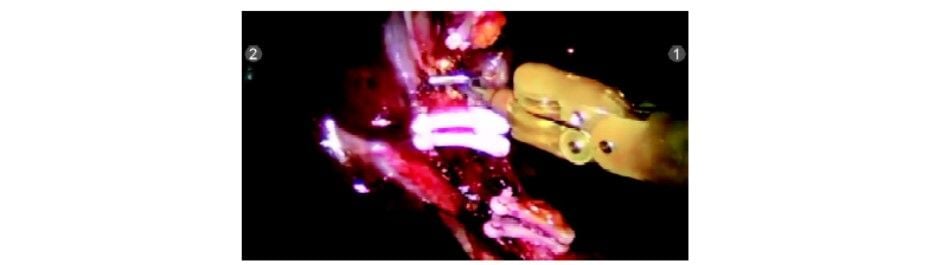
Cystic artery being divided with Endowrist hook
Step 7
Cystic duct divided

Cystic artery dissected

Clip applied to cystic artery

Cystic artery being divided with Endowrist hook
The gallbladder is dissected off the gallbladder (GB) fossa with the help of a hook electrode with monopolar cautery (R1). The Endowrist function of the hook provides a lot of versatile movements that aid in this step of surgery.

Dissection of GB off GB fossa commenced
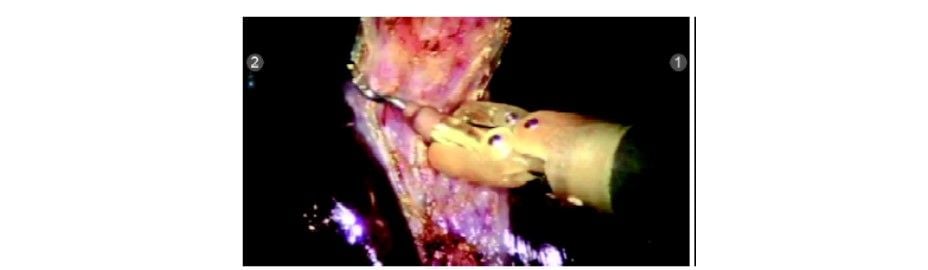
Dissection did in the avascular plane
Step 8
Dissection did in the avascular plane
Just before taking off the gallbladder from the fossa, it is retracted cephalad and the GB fossa is inspected for any bleed or bile leak.
 Inspection of GB fossa
Inspection of GB fossaStep 9
The R3 arm is undocked and endobag is inserted by the patient side assistant surgeon using a standard laparoscopic grasper. Any suction/irrigation can also be done by the assistant surgeon by undocking R3. The gallbladder is placed in the endobag and extracted out.

Gallbladder placed in endobag
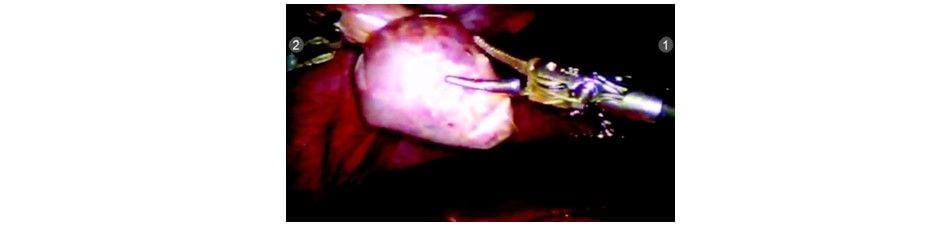

GB being extracted out
Step 10
Check for hemostasis and clip position. Remove instruments and ports under vision. Incisions are closed in layers.
Discussion
Laparoscopic cholecystectomy for the treatment of biliary colic is a relatively straightforward, routine procedure. It will be difficult to defend the use of a highly developed and expensive surgical tool that requires additional time for preparation and larger incisions for the trocars, especially while the advantages of such tools are hard to prove for this specific procedure.
There are two situations in which robotics can be applied to this particular procedure:
1. Complicated gallstone disease, like acute cholecystitis, CBD exploration or choledochoenterostomy.
2. Ideal learning and teaching environment for gastrointestinal robot-assisted surgery.
Advantages
Robotic instruments clearly provide superiority in some aspects of the surgery. The increased degrees of freedom (wrist action) allow the surgeon to easily reach behind structures and negotiate difficult surgical angles. The ergonomics of the operating surgeon can be significantly improved by operating in the sitting position and facing directly forward toward the 3D operative image. These can be of value in cases of acute and chronic cholecystitis, where vision is impaired by inflammation, where there is an increased tendency for diffuse bleeding in the dissection field, and edema and fibrosis make the dissection difficult.
By adjusting movement scaling, surgeon tremors can be significantly reduced; thereby, increasing operative precision. The translation of the surgeon’s hand and wrist movements are reliable and can be scaled down to improve precision and increase steadiness.
Limitations
There is a need for more variety in the type and size of robotic trocars. The robotic arms are bulky, taking up significant space and limiting the capability of the surgical assistant. Furthermore, the da Vinci system is not attached to the operating table, so the position of the table cannot be changed without undocking the instrument and camera. The robotic arms lack haptic feedback, so the surgeon must rely on visual cues to avoid stretching and damaging tissue or suture. Financially, surgical robotic systems remain quite expensive and are associated with significant operating costs. Current robotic systems and instrumentation are still considered to be first-generation and should improve as the technology evolves.
Conclusion
Robotic cholecystectomy is feasible and safe. There is a significant learning curve (around 20–30 cases) to gain experience for setting-up the robotic instrumentation, which appears to be much less steep for the actual use of the machine.
It is unlikely that robotic cholecystectomy will be routinely performed in the near future. Further studies are needed to identify the benefits to the patient and compare it to the additional cost of robotic cholecystectomy before routine application of this technique can be justified. However, robotic cholecystectomy may prove its value in cases of complex gallstone disease and is an excellent procedure for teaching the basics of robotic surgery.
Robotic Coronary Bypass Surgery
Four ports are used and a robotic arm is introduced. One of the arms contains a telescope which sends 12 to 15 times a magnified clear image on the monitor. Another arm is for a stabilizer which is used to hold the diseased coronary artery in place while bypass is performed. The other two remaining right and the left instrument is used to perform the microvascular anastomosis. Currently, robotic coronary bypass surgery should be considered only for the patient who has single-vessel disease but in near future, we have hope to perform double or even triple bypass surgery.
The attached instruments are controlled by the surgeon, who sits at an adjacent console. Several passive mechanical devices, primarily used to hold the telescope, have been developed as assistants for general laparoscopic surgery. They successfully reduce the stress on the surgeon by eliminating the inadvertent movements of a human assistant, which can be distracting and disorienting.
More and more surgeries from the prostate to the heart are being performed by doctors remotely guiding robotic arms. In such procedures, the surgeon’s hands never enter the patient. After the initial incisions are made, robotic arms wielding a tiny camera and surgical tools make the snips, stanch the blood flow, and sew up inside when all is done. The surgeon sits at a console usually in the operating room, although the technology would allow a doctor to operate on a patient on the other side of the world peering into binocular-like lenses at views provided by the camera inside the patient. The doctor guides the robot’s work by twisting his wrists in the stirrup- like handles, moving his thumb and forefinger in scissor-like loops, or tapping foot pedals to focus the camera or move a robotic arm.

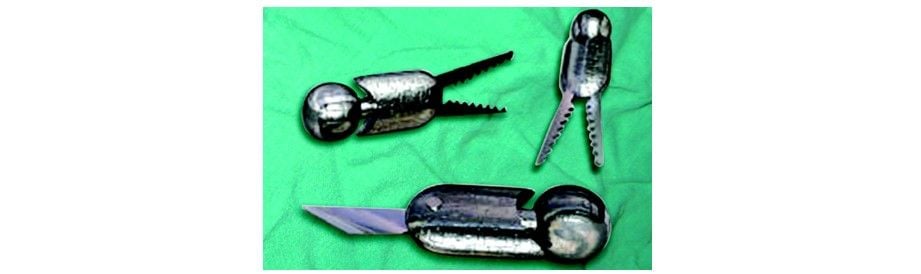
Instruments used by the da Vinci surgical system
Robotic Prostatectomy
The most common robotic surgery is radical prostatectomy. Its use has grown from just 1,500 procedures in 2000 to an estimated 20,000 last year. More than 8,000 prostate glands were removed robotically last year, up from 36 in 2000. The procedure accounted for more than 10 percent of the 75,000 prostatectomies done in 2004. Busy professionals like the fact that they can be out of the hospital in a day, vs. two or three for open surgery, and resume normal activities in one week rather than six. Cutting nerves around the prostate can lead to incontinence or impotence, so precision is important. Between 25 and 60 percent of conventional prostatectomy patients suffer from postoperative impotence. Small studies of robotic surgery have shown at least a short-term benefit in terms of impotence and incontinence.
Not every patient is a candidate. Complicated cases when the patient is very sick, need multiple procedures, or has had previous chest surgery are not suited to robotics. Nor can a heart transplant or artificial heart implant be done this way. If the complication arises, the patient may wind-up being opened. So patients who want robotics smaller scars and quicker recovery times will need to ask hard questions. Find out how many times, the procedure you need has been done robotically and what the advantages and disadvantages are. Just as important, ask how many robotic surgeries, the doctor who will do yours has performed. In real life, you want the force of experience to be with you.
For qualified candidates, the robotic prostatectomy offers numerous potential benefits over the traditional open prostatectomy, including:
• Shorter hospital stay
• Less pain
• Less risk of infection
• Less blood loss and transfusions
• Less scarring
• Faster recovery
• Quicker return to normal activities.

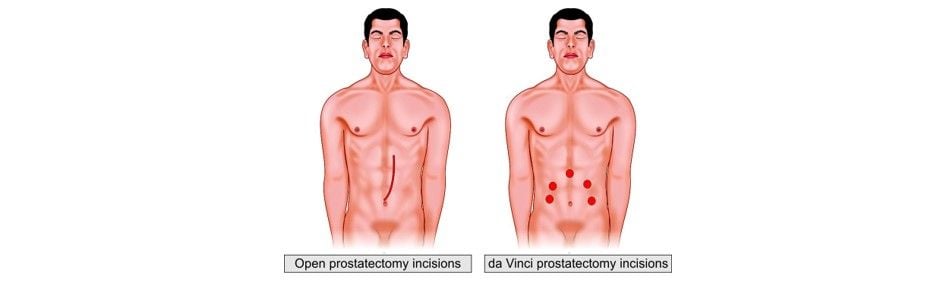
Comparison between open and da Vinci prostatectomy
While still a technology that is in its infancy, the use of robots to assist in surgery is becoming more and more widespread. There are now large case series reported in the literature that show possible benefits to the patient in terms of recovery from such surgeries as prostate and cardiac procedures. Additionally, as these systems continue to develop, improved technology and software provide the surgeon with “assistance” that improves precision and accuracy.
Robotic technology requires a tremendous financial investment, so you might not see it at every community hospital in the near future. However, as with all technology, the price will likely fall quickly as the applications are expanded, as more widespread adoption occurs, and as the field of robotics experiences additional breakthroughs. For now, many major academic institutions are beginning to purchase and deploy these systems.
Technology allows for amazing things to be accomplished. Robotic surgery is a new and expensive tool that is beginning to see adoption. Minimally invasive surgery with a robot and without a bypass is the next logical step in the development of cardiac surgery. It is beginning to show clear benefits for patients and this means that it will likely become more and more popular with time and as the price falls.

Robotic-assisted nephrectomy
Robotic Versus Laparoscopic Prostatectomy
In laparoscopy, the surgeon uses long instruments through small openings and maneuvers them with direct hand contact. Robotic systems use even more delicate instruments that possess two additional degrees of movement excursion (for a total of six, as with a human hand). Comfortably seated at the robot console, the surgeon can maneuver the instruments via a computer interface.
Laparoscopic radical prostatectomy is associated with a steep learning curve. Even in the hands of expert surgeons, laparoscopic radical prostatectomy requires extensive learning; approximately 40 cases are needed to master this technique. In contrast, the learning of robotic prostatectomy seems to be more intuitive and less demanding.
Robotic prostatectomy is a safe, effective, and reproducible technique for removing the prostate. In most patients, it can be performed within one and a half to two hours with minimal blood loss and few complications. The procedure incorporates principles of both laparoscopic and open radical prostatectomy. The patients enjoy the benefits of surgical treatment in the setting of less invasion, minimal pain, limited blood loss, and early functional and overall recovery.





