Laparoscopic Management of Abdominal Aortic Aneurysm (AAA)
Introduction:
o Definition:
An abdominal aorta with a maximal diameter >3.0 cm is considered aneurysmal in most adult patients (1, 2, 3) also an increment of the vessel diameter by one and half times of the original diameter is an aneurysm. Fig 1.
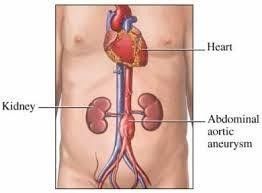
Fig 1: dilatation of distal aorta.(doctorstock.photoshelter.com/image/1000wDREu2ZfEnc)
Abdominal aortic aneurysm (AAA) most often infrarenal, approximately 5 percent involve the renal (pararenal) or visceral arteries (suprarenal) (4).
Abdominal aortic aneurysm is a common and potentially life-threatening condition. Those ruptured ones are almost uniformly fatal, 50 percent die before reaching the hospital, the other 50 percent of patients with ruptured AAA who reach the hospital for treatment, between 30 and 50 percent will die in the hospital (5, 6).
Elective repair is the gold standard and the most effective management to prevent rupture, Although, elective surgical intervention is considered as a major surgery that is associated with risks, for this reason asymptomatic patient are not indicated for surgery till the risk of rupture exceeds the risk of surgery.
Elective repair can be an open surgical procedure or endovascular stinting, and the choice between them is still controversial, however, those patients who are not fit for surgery may tolerate endovascular stinting, while medical management is just for those who are not indicated for repair aiming to reduce the vascular events (thromboembolism), and limiting the rate of aneurysm distension.
Classification:
AAA can be classified according to the aetiology, shape, and size, aetiology is not included here.
o Shape:
Fusiform.
Saccular.
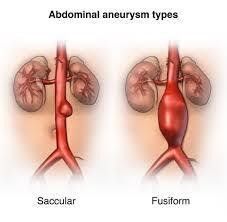
Fig 2: shapes of aneurysm, (www.hopkinsmedicine.org)
o Size:
Small aneurysms have a diameter <4.0 cm.
Medium aneurysms have a diameter between 4.0 and 5.5 cm.
Large aneurysms have a diameter ≥5.5 cm.
Very large aneurysms have a diameter ≥6.0 cm.
Rapid expansion, which is thought to increase the risk for rupture, is defined as an increase in maximal aortic diameter ≥5 mm over a six-month period of time or >10 mm over a year, using the same radiographic method of measurement (2).
The Objective:
The primary objective is a minimal access surgery that may help in the management of AAA, aiming to reduce its expansion and risk of rupture, secondary objective is to expand the field of minimal access surgery.
Methodology:
This a new technique used single incision laparoscopic surgery (SILS) in the management of AAA. It’s been done on animal (pig) for demonstration and explanation of the procedure despite the animal has no aneurysm. So till now it’s theoretical and not applied to humans yet.
Inclusions:
This procedure is dealing with small, and medium sized aneurysms, both fusiform and saccular including rapidly expanding ones regardless the aetiology.
Exclusions:
The large aneurysms are excluded, also this procedure is not applicable, for emergency (rupture).
Procedure:
*The used instrument are SILS instrument (the articulating ones).
*New designed port to allow the bringing of the clip into the abdomen.
*The clip which surround the aneurysm preventing its expansion without interruption to the blood flow.
SILS Instrument:
.Scissor.
.Maryland.
.Crocodile or grasper forceps.
.Camera.
The Port:
*The port is a tube like with a diameter of 3cm or more, and inlet and outlet cover that can be opened and closed alternately to allow the admission of the clip into the abdomen without losing air (similar to submarine protection against water). Fig 3, 4 also can be used in tissue retrieval in other operation especially caner to bring out a complete specimen.
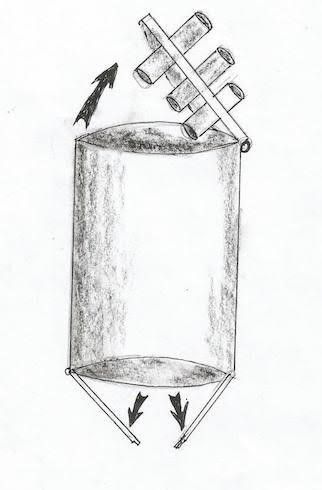
Fig 3: The port, schematic draw shows the port with inlet and out let, and the way they open and close.
*The port inlet has small holes for the passage of the instrument and the camera (5mm).Fig 3, 4, and 5
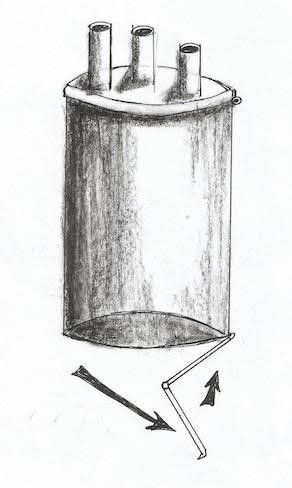
Fig 4: The schematic draw of the port and its out let is opening with different way compared to fig 3, while inlet is closed.
*The outlet has no holes, simply can be just opened during the operation not to embed the instrument movement (the lever is the inlet), and can be closed just to prevent air loss during in/out clip delivery. Fig 3, 4.
** The port diameter can be increased during the operation by tilting it, because its skeleton working like stent. So increasing the diameter and reducing the length without any problem in the diameter of inlet and outlet because it’s supposed to be made of rubber, then increase the diameter of abdominal port site without increasing the incision (elastic propriety of skin) can be used instead of reducer other laparoscopic surgery. Fig 5

Fig 5: The skeleton of the port. Adjustable to change its diameter.
*The incision is basically through anterior abdominal wall, and the dissection will be directly over the aneurysm, exposing the aneurysm, and identification its branches, to make it easy to be surrounded by the clip, and held between its arms and fingers without interfering with the branches and to keep them all intact.
The Clip:
*The clip is essentially made of silicon, and opened against resistance that will be spontaneously closed when left to cover the aneurysm without reducing the aneurysm size, just overlying it, and secured with a lock. It has finger-like projections with gaps in between allowing spaces for any aortic branch and not to disrupt the blood supply, (Using a hair clip for demonstration and explanation). Fig 6, 7

Fig.6 A: gap between clip fingers. B: grasper

Fig. 7 the clip is opened
*The clip designed to have 2 arms in order to be held by grasper or crocodile and bring them together to be opened against the resistance which separate the arms to keep the clip closed, the movement of the arms move the hands in the opposite direction on a joint connecting them together as the main body of the clip, each hand has 4 or more fingers.
*The clips are in different sizes, and different diameters when they are closed the smallest one is 3cm.Fig. 8
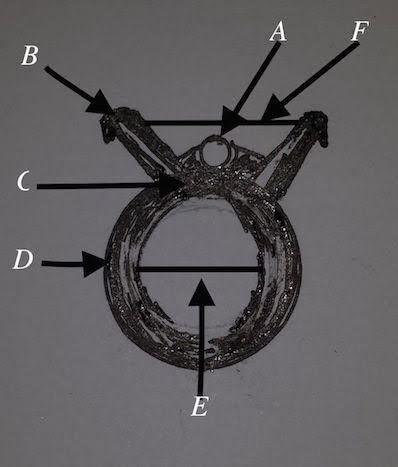
Fig 8
*A special lock can be used to keep the clip closed against the suspected expansion of the aneurysm. Fig 9
**The fenestrated metallic stent can be implanted from inside against the inwardly inserted clip from the outside to provide support from both sides, in order to prevent pressure necrosis on the vasculature wall, such technique will provide low pressure between the clip and the stent structure. The idea from making the stent fenestrated is to keep the aortic branches intact, and there is no endoleak. (Optional).
*The incision site is midline through the anterior abdominal wall in the midway between umbilicus and xiphisternum, or to the right side of the abdomen. *Creating a pneumoperitonium followed by direct dissection of mesentery over the aneurysm, identifying the branches to preserve them, then all instruments will be taken out the peritoneal cavity, choosing the appropriate clip size then opening just the inlet of the port and through the clip inside the port cavity, and closing the the inlet followed by opening the port outlet to allow the clip to fall in the peritoneal cavity without losing the pneumoperitonium. The instruments and camera returned back to port hols, holding the clip, opening it and applied it over the aneurysm, if more than one clip is needed, then the same thing can be done, or just in one step deliver more than one clip to the peritoneal cavity. Fig 9,10, and 11
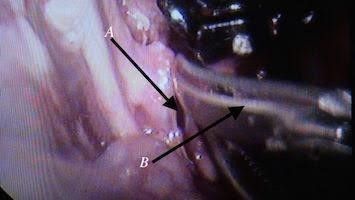
Fig. 9 Dissection to expose the aorta, A: mesentery over aorta, B: scissor
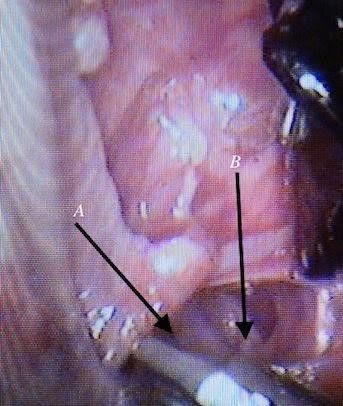
Fig.10 Dissection around the aorta, A: aorta, B: inferior vena cava
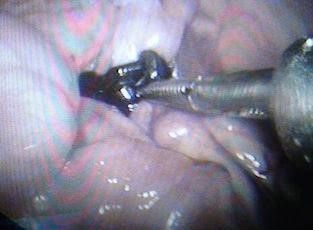
Fig. 11: Applying a clip over the aorta.
*The gap between the fingers like projections in the clip is designed to give space for aortic branches and not to interfere with other organ blood supply (to avoid the risk of ischemia e.g.; inferior mesenteric artery, or vertebral arteries). Fig12, 13, and 14
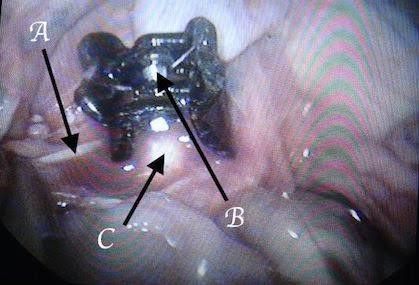
Fig. 12 A: inferior mesenteric artery, B: clip resistance, C: aorta
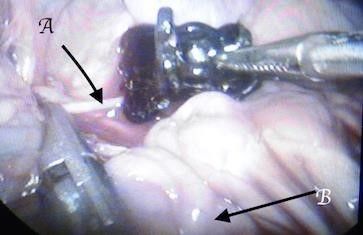
Fig. 13: Keeping the inferior mesenteric intact between the clip fingers. A: inferior mesenteric artery, B: small bowel
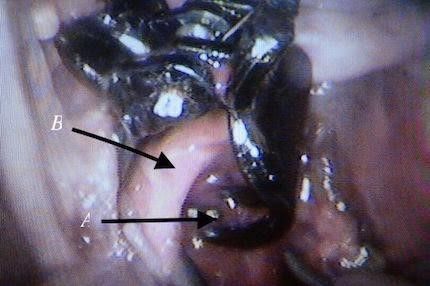
Fig. 14 Clip fingers pass behind the aorta inclosing it without interfere with the vertebral branches, A: clip finger from behind, B: aorta
*The aneurysm is completely surrounded by the clip/clips, without reducing its diameter, at the same time preventing its expansion.Fig14, 15, and 16
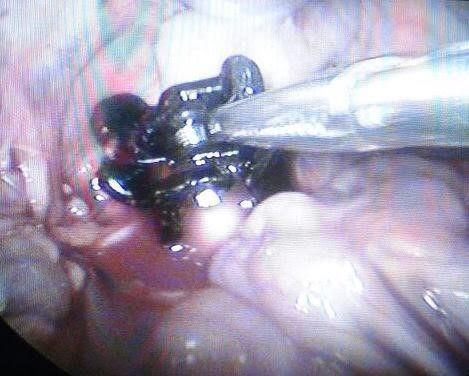
Fig.15: The clip completely covered the aorta
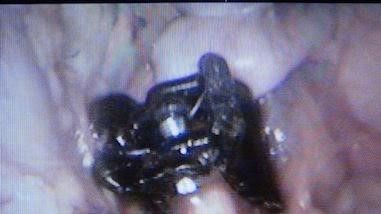
Fig. 16: *Closing the mesentery over the clip and leaving it in situ.
Current Management:
There are different modalities for the management of AAA according to indications and patient situations where best treatment is chosen, and here we are not dealing with the rupture AAA, so the discussion will be for elective repair including the new procedure:
Fig 17, (lifelikebiotissue.com/)
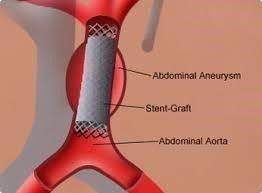
Fig 18, (www.uchopitals.edu)
Discussion:
It’s clear from the above that minimal access surgery (MAS) is rapidly growing and replacing the classical open one, however, open surgery still strong in certain situation where MAS failed.
In the case of abdominal aortic aneurysm repair, no doubt that open surgery is the gold standard option in dealing with emergency situations as ruptured aneurysms.
In certain situation open surgery can’t be done due to patient morbidity whom unable to tolerate such a major surgery.
EVAR is the best option when the patient is not fit for anaesthesia or surgery and the stent can be implanted under local anaesthesia, much more its less invasive, and less stay in the hospital, on the other hand it’s not devoid of complication, like endoleak where the blood can scape between the vessel (sac) wall, and the outer cover of the stent, which supposed to be separated from the circulation, also in case of separation and no endoleak the branches arising at the lined area will cut-off with ischemia to the area supplied by them.
Other complication as mentioned above are associated with the stenting. For this reason the clip is designed to be made of silicon to reduce the body reaction, and must by applied from outside the sac to reduce the blood branches cut of and ischemia.
Laparoscopic repair proved to be effective as well as open repair, with less invasion, short hospitalisation, and low risk of renal impairment, but taking long time and technically is more difficult. On the other hand the new procedure is technically easy, and of short duration of time, also using one small incision (SILS).
Randomized trials comparing open AAA repair with EVAR have found significantly improved perioperative (30-day) morbidity and mortality for EVAR, but no significant differences in long-term outcomes (10,11,12). A pooled analysis of these trials identified a 69% reduction in the risk for perioperative mortality for endovascular compared with open repair (13). EVAR appears to be associated with the need for more secondary procedures, and an ongoing future risk of aortic rupture. However, there appears to be no significant differences in overall complication rates when all complications of each procedure are accounted for (14).
The clip can be used with uncovered stent to hold it in place, without interrupting the branches, and with covered stent just at both ends to reduce the risk of endoleak, keeping in mind the pressure between the two is not big enough to cause the pressure necrosis of the aortic wall.
Such new technique never been tested in humans and no previous data up to date to identify up to what extent that the patient can get benefit out from it, However, the operation technically is not difficult, does not last long time, part of minimal access surgery, i.e.; short hospital stay, and the new designed ports can be used in other surgeries.
Conclusion:
This is a new technique using laparoscopy (MAS) in the management of medium and small size AAA, although, till now it’s not done on humans, we recommend this procedure to be one of the options used in the AAA management and to find out its benefit and disadvantage, also the new port is very helpful in the MAS field.
Refrences:
1. Wanhainen. A. How to define an abdominal aortic aneurysm-influence on epidemiology and clinical practice. Scand J Surg 2008; 97:105
2. Johnston KW, Rutherford RB, Tilson MD, et al. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg 1991; 13:452.
3. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines
(Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial
Disease): endorsed by American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; Trans Atlantic Inter Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113:e463.
4. Jongkind V, Yeung KK, Akkersdijk GJ, et al. Juxtarenal aortic aneurysm repair. J Vasc Srug 2010;52:760.
5. Hoomweg LL, Storm-Versloot MN, Ubbink DT, et al. Meta analysis on mortality of ruptured abdominal aortic aneurysm. Eur J Vas Endovasc Surg 2008; 35:558.
6. Bown MJ, Sutton AJ, Bell PR, SayersRD. A meta-analysis of 50 years of ruptured abdominal aortic aneurysm repair. Br J Surg 2002; 89:714.
7. Isselbacher EM. Thoracic and abdominal aortic aneurysm. Circulation 2005; 111:816.
8. Bergggvist D, Pharmacological interventions to attenuate the expansion of abdominal aortic aneurysm-a systematic review. Eur J Vac Endovasc Surg 2011;41:663.
9. Assar AN, Medical treatment of small abdominal aortic aneurysm. J Cardiovasc Surg (Torino) 2012;53:517.
10. United Kingdom EVAR Trial Investigators, Greenhalg RM, Brown LC, et al. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med 2010;632:1863.
11. Brown LC, Thompson SG, Greenhalg RM, et al. Incidence of cardiovascular events and death after open or endovascular repair of abdominal aortic aneurysm in the randomized EVAR trial 1.BR J Surg 2011;98;935.
12. Stather PW, Sidloff D, Dattani N, et al.Systematic review and meta-analysis of the early and late outcomes of open and endovascular repair of abdominal aortic aneurysm. Br J Surg 2013;100:863.
13. Karthikesalingam A, Thompson MM. Vascular disease: Repair of infrarenal aortic aneurysm-the debate is over. Nat Rev Cardiol 2013;10:122.
14. Schermerhorn ML, O’Malley AJ, Jhaveri A, et al. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med 2008;358:464.
o Definition:
An abdominal aorta with a maximal diameter >3.0 cm is considered aneurysmal in most adult patients (1, 2, 3) also an increment of the vessel diameter by one and half times of the original diameter is an aneurysm. Fig 1.

Fig 1: dilatation of distal aorta.(doctorstock.photoshelter.com/image/1000wDREu2ZfEnc)
Abdominal aortic aneurysm (AAA) most often infrarenal, approximately 5 percent involve the renal (pararenal) or visceral arteries (suprarenal) (4).
Abdominal aortic aneurysm is a common and potentially life-threatening condition. Those ruptured ones are almost uniformly fatal, 50 percent die before reaching the hospital, the other 50 percent of patients with ruptured AAA who reach the hospital for treatment, between 30 and 50 percent will die in the hospital (5, 6).
Elective repair is the gold standard and the most effective management to prevent rupture, Although, elective surgical intervention is considered as a major surgery that is associated with risks, for this reason asymptomatic patient are not indicated for surgery till the risk of rupture exceeds the risk of surgery.
Elective repair can be an open surgical procedure or endovascular stinting, and the choice between them is still controversial, however, those patients who are not fit for surgery may tolerate endovascular stinting, while medical management is just for those who are not indicated for repair aiming to reduce the vascular events (thromboembolism), and limiting the rate of aneurysm distension.
Classification:
AAA can be classified according to the aetiology, shape, and size, aetiology is not included here.
o Shape:
Fusiform.
Saccular.

Fig 2: shapes of aneurysm, (www.hopkinsmedicine.org)
o Size:
Small aneurysms have a diameter <4.0 cm.
Medium aneurysms have a diameter between 4.0 and 5.5 cm.
Large aneurysms have a diameter ≥5.5 cm.
Very large aneurysms have a diameter ≥6.0 cm.
Rapid expansion, which is thought to increase the risk for rupture, is defined as an increase in maximal aortic diameter ≥5 mm over a six-month period of time or >10 mm over a year, using the same radiographic method of measurement (2).
The Objective:
The primary objective is a minimal access surgery that may help in the management of AAA, aiming to reduce its expansion and risk of rupture, secondary objective is to expand the field of minimal access surgery.
Methodology:
This a new technique used single incision laparoscopic surgery (SILS) in the management of AAA. It’s been done on animal (pig) for demonstration and explanation of the procedure despite the animal has no aneurysm. So till now it’s theoretical and not applied to humans yet.
Inclusions:
This procedure is dealing with small, and medium sized aneurysms, both fusiform and saccular including rapidly expanding ones regardless the aetiology.
Exclusions:
The large aneurysms are excluded, also this procedure is not applicable, for emergency (rupture).
Procedure:
*The used instrument are SILS instrument (the articulating ones).
*New designed port to allow the bringing of the clip into the abdomen.
*The clip which surround the aneurysm preventing its expansion without interruption to the blood flow.
SILS Instrument:
.Scissor.
.Maryland.
.Crocodile or grasper forceps.
.Camera.
The Port:
*The port is a tube like with a diameter of 3cm or more, and inlet and outlet cover that can be opened and closed alternately to allow the admission of the clip into the abdomen without losing air (similar to submarine protection against water). Fig 3, 4 also can be used in tissue retrieval in other operation especially caner to bring out a complete specimen.
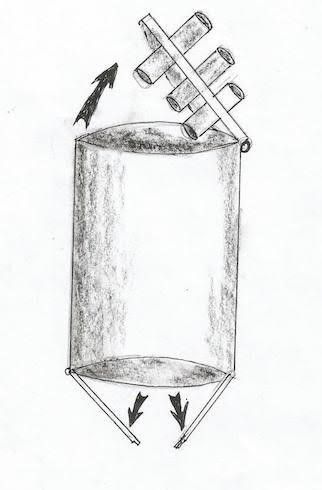
Fig 3: The port, schematic draw shows the port with inlet and out let, and the way they open and close.
*The port inlet has small holes for the passage of the instrument and the camera (5mm).Fig 3, 4, and 5

Fig 4: The schematic draw of the port and its out let is opening with different way compared to fig 3, while inlet is closed.
*The outlet has no holes, simply can be just opened during the operation not to embed the instrument movement (the lever is the inlet), and can be closed just to prevent air loss during in/out clip delivery. Fig 3, 4.
** The port diameter can be increased during the operation by tilting it, because its skeleton working like stent. So increasing the diameter and reducing the length without any problem in the diameter of inlet and outlet because it’s supposed to be made of rubber, then increase the diameter of abdominal port site without increasing the incision (elastic propriety of skin) can be used instead of reducer other laparoscopic surgery. Fig 5

Fig 5: The skeleton of the port. Adjustable to change its diameter.
*The incision is basically through anterior abdominal wall, and the dissection will be directly over the aneurysm, exposing the aneurysm, and identification its branches, to make it easy to be surrounded by the clip, and held between its arms and fingers without interfering with the branches and to keep them all intact.
The Clip:
*The clip is essentially made of silicon, and opened against resistance that will be spontaneously closed when left to cover the aneurysm without reducing the aneurysm size, just overlying it, and secured with a lock. It has finger-like projections with gaps in between allowing spaces for any aortic branch and not to disrupt the blood supply, (Using a hair clip for demonstration and explanation). Fig 6, 7

Fig.6 A: gap between clip fingers. B: grasper

Fig. 7 the clip is opened
*The clip designed to have 2 arms in order to be held by grasper or crocodile and bring them together to be opened against the resistance which separate the arms to keep the clip closed, the movement of the arms move the hands in the opposite direction on a joint connecting them together as the main body of the clip, each hand has 4 or more fingers.
*The clips are in different sizes, and different diameters when they are closed the smallest one is 3cm.Fig. 8

Fig 8
*A special lock can be used to keep the clip closed against the suspected expansion of the aneurysm. Fig 9
**The fenestrated metallic stent can be implanted from inside against the inwardly inserted clip from the outside to provide support from both sides, in order to prevent pressure necrosis on the vasculature wall, such technique will provide low pressure between the clip and the stent structure. The idea from making the stent fenestrated is to keep the aortic branches intact, and there is no endoleak. (Optional).
*The incision site is midline through the anterior abdominal wall in the midway between umbilicus and xiphisternum, or to the right side of the abdomen. *Creating a pneumoperitonium followed by direct dissection of mesentery over the aneurysm, identifying the branches to preserve them, then all instruments will be taken out the peritoneal cavity, choosing the appropriate clip size then opening just the inlet of the port and through the clip inside the port cavity, and closing the the inlet followed by opening the port outlet to allow the clip to fall in the peritoneal cavity without losing the pneumoperitonium. The instruments and camera returned back to port hols, holding the clip, opening it and applied it over the aneurysm, if more than one clip is needed, then the same thing can be done, or just in one step deliver more than one clip to the peritoneal cavity. Fig 9,10, and 11

Fig. 9 Dissection to expose the aorta, A: mesentery over aorta, B: scissor

Fig.10 Dissection around the aorta, A: aorta, B: inferior vena cava

Fig. 11: Applying a clip over the aorta.
*The gap between the fingers like projections in the clip is designed to give space for aortic branches and not to interfere with other organ blood supply (to avoid the risk of ischemia e.g.; inferior mesenteric artery, or vertebral arteries). Fig12, 13, and 14

Fig. 12 A: inferior mesenteric artery, B: clip resistance, C: aorta

Fig. 13: Keeping the inferior mesenteric intact between the clip fingers. A: inferior mesenteric artery, B: small bowel

Fig. 14 Clip fingers pass behind the aorta inclosing it without interfere with the vertebral branches, A: clip finger from behind, B: aorta
*The aneurysm is completely surrounded by the clip/clips, without reducing its diameter, at the same time preventing its expansion.Fig14, 15, and 16

Fig.15: The clip completely covered the aorta

Fig. 16: *Closing the mesentery over the clip and leaving it in situ.
Current Management:
There are different modalities for the management of AAA according to indications and patient situations where best treatment is chosen, and here we are not dealing with the rupture AAA, so the discussion will be for elective repair including the new procedure:
- Medical management focusing on modifiable risk factors to reduce the expansion of the aneurysm, reduce the cardiovascular mortality and morbidity (7), and reduce the risk of thrombosis. A number of drug targets that may inhibit AAA formation or progression have been identified in animal models and in vitro studies, looking for treatment of patients with small aneurysms; although, there is no proven evidence that any of these targets unequivocally prevents aortic expansion and rupture (8-9); including: statins, anti-platelets, anti-inflammatory, beta blockers, etc., and life style modification especially cessation of smoking.
- Open surgical repair involves replacement of the aneurysm with a prosthetic graft. Complications such as acute renal failure, distal embolization, wound infection, colonic ischemia, false aneurysm formation, aorto-duodenal fistula, graft infection, and spinal cord ischemia can be happened. Fig 17
- Laparoscopic surgical repair can be done by hand assisted laparoscopic surgery( HALS) or totally laparoscopic surgery, with the same complication of open surgery, the main differences are less invasive and less hospital stay, and more time consuming, also technically more difficult, and less renal impairment.
- EVAR – Endovascular aneurysm repair (EVAR) involves the placement of covered metallic stent delivered via the femoral artery to line the aneurysm and exclude the sac from the circulation. Although EVAR is associated with lower perioperative mortality, late AAA rupture has been reported. Migration, fracture, and infection of the stent are part of complications as well as endoleak. Fig 18
- Multilayered stent which is still under trail.

Fig 17, (lifelikebiotissue.com/)

Fig 18, (www.uchopitals.edu)
Discussion:
It’s clear from the above that minimal access surgery (MAS) is rapidly growing and replacing the classical open one, however, open surgery still strong in certain situation where MAS failed.
In the case of abdominal aortic aneurysm repair, no doubt that open surgery is the gold standard option in dealing with emergency situations as ruptured aneurysms.
In certain situation open surgery can’t be done due to patient morbidity whom unable to tolerate such a major surgery.
EVAR is the best option when the patient is not fit for anaesthesia or surgery and the stent can be implanted under local anaesthesia, much more its less invasive, and less stay in the hospital, on the other hand it’s not devoid of complication, like endoleak where the blood can scape between the vessel (sac) wall, and the outer cover of the stent, which supposed to be separated from the circulation, also in case of separation and no endoleak the branches arising at the lined area will cut-off with ischemia to the area supplied by them.
Other complication as mentioned above are associated with the stenting. For this reason the clip is designed to be made of silicon to reduce the body reaction, and must by applied from outside the sac to reduce the blood branches cut of and ischemia.
Laparoscopic repair proved to be effective as well as open repair, with less invasion, short hospitalisation, and low risk of renal impairment, but taking long time and technically is more difficult. On the other hand the new procedure is technically easy, and of short duration of time, also using one small incision (SILS).
Randomized trials comparing open AAA repair with EVAR have found significantly improved perioperative (30-day) morbidity and mortality for EVAR, but no significant differences in long-term outcomes (10,11,12). A pooled analysis of these trials identified a 69% reduction in the risk for perioperative mortality for endovascular compared with open repair (13). EVAR appears to be associated with the need for more secondary procedures, and an ongoing future risk of aortic rupture. However, there appears to be no significant differences in overall complication rates when all complications of each procedure are accounted for (14).
The clip can be used with uncovered stent to hold it in place, without interrupting the branches, and with covered stent just at both ends to reduce the risk of endoleak, keeping in mind the pressure between the two is not big enough to cause the pressure necrosis of the aortic wall.
Such new technique never been tested in humans and no previous data up to date to identify up to what extent that the patient can get benefit out from it, However, the operation technically is not difficult, does not last long time, part of minimal access surgery, i.e.; short hospital stay, and the new designed ports can be used in other surgeries.
Conclusion:
This is a new technique using laparoscopy (MAS) in the management of medium and small size AAA, although, till now it’s not done on humans, we recommend this procedure to be one of the options used in the AAA management and to find out its benefit and disadvantage, also the new port is very helpful in the MAS field.
Refrences:
1. Wanhainen. A. How to define an abdominal aortic aneurysm-influence on epidemiology and clinical practice. Scand J Surg 2008; 97:105
2. Johnston KW, Rutherford RB, Tilson MD, et al. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg 1991; 13:452.
3. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines
(Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial
Disease): endorsed by American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; Trans Atlantic Inter Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113:e463.
4. Jongkind V, Yeung KK, Akkersdijk GJ, et al. Juxtarenal aortic aneurysm repair. J Vasc Srug 2010;52:760.
5. Hoomweg LL, Storm-Versloot MN, Ubbink DT, et al. Meta analysis on mortality of ruptured abdominal aortic aneurysm. Eur J Vas Endovasc Surg 2008; 35:558.
6. Bown MJ, Sutton AJ, Bell PR, SayersRD. A meta-analysis of 50 years of ruptured abdominal aortic aneurysm repair. Br J Surg 2002; 89:714.
7. Isselbacher EM. Thoracic and abdominal aortic aneurysm. Circulation 2005; 111:816.
8. Bergggvist D, Pharmacological interventions to attenuate the expansion of abdominal aortic aneurysm-a systematic review. Eur J Vac Endovasc Surg 2011;41:663.
9. Assar AN, Medical treatment of small abdominal aortic aneurysm. J Cardiovasc Surg (Torino) 2012;53:517.
10. United Kingdom EVAR Trial Investigators, Greenhalg RM, Brown LC, et al. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med 2010;632:1863.
11. Brown LC, Thompson SG, Greenhalg RM, et al. Incidence of cardiovascular events and death after open or endovascular repair of abdominal aortic aneurysm in the randomized EVAR trial 1.BR J Surg 2011;98;935.
12. Stather PW, Sidloff D, Dattani N, et al.Systematic review and meta-analysis of the early and late outcomes of open and endovascular repair of abdominal aortic aneurysm. Br J Surg 2013;100:863.
13. Karthikesalingam A, Thompson MM. Vascular disease: Repair of infrarenal aortic aneurysm-the debate is over. Nat Rev Cardiol 2013;10:122.
14. Schermerhorn ML, O’Malley AJ, Jhaveri A, et al. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med 2008;358:464.