Development of Novel Infra-Red Gastric Calibration Tube to Improve Performance in Gastric Plication And Laparoscopic Sleeve Gastrectomy
Dr. Hanumanth Ammanna Ammanna
MS. General Surgery; M.MAS
MS. General Surgery; M.MAS
INTRODUCTION
Obesity is associated with multiple adverse effects on health which can be improved with weight loss. Limited long-term success of behavioural and pharmacological therapies in patients with severe obesity has led to increased interest in surgical options in recent times namely sleeve gastrectomy, plication of greater curvature of stomach, roux – en- y gastric by-pass, biliopancreatic diversion with duodenal switch and etc1. All these procedures may result in significant weight loss in well-informed and motivated patients with higher BMI ( > 35)1. These procedures involve making changes to your digestive system. Some procedures limit how much you can eat, while others work by reducing the body's ability to absorb nutrients and some procedures achieve both. Gastric bougie used in bariatric procedures helps to calibrate the stomach during resection of the stomach as in sleeve gastrectomy and prevent excessive narrowing near incisura and also helps assessing proper closure of hiatus hernia2. Plication of the greater curvature of the stomach simulates the results of laparoscopic sleeve gastrectomy but with fewer complications3. Near-infrared (NIR) light penetrates relatively deeper into biological soft tissues but varies with tissue constituents such as water, fat, collagen, and their combination ratio4. The aim of the study was to apply the near infra-red-light property to a conventional gastric bougie and understand its role during surgery.
MATERIALS AND METHODS
PLACE OF STUDY
The study was done at world laparoscopy hospital, from the department of Minimal Access Surgery with prior pilot study done on animal after approval from the Committee F or the Purpose of Control and Supervision of Experiments on Animals on 25th September 2019 (Approval number- 25/17/209).
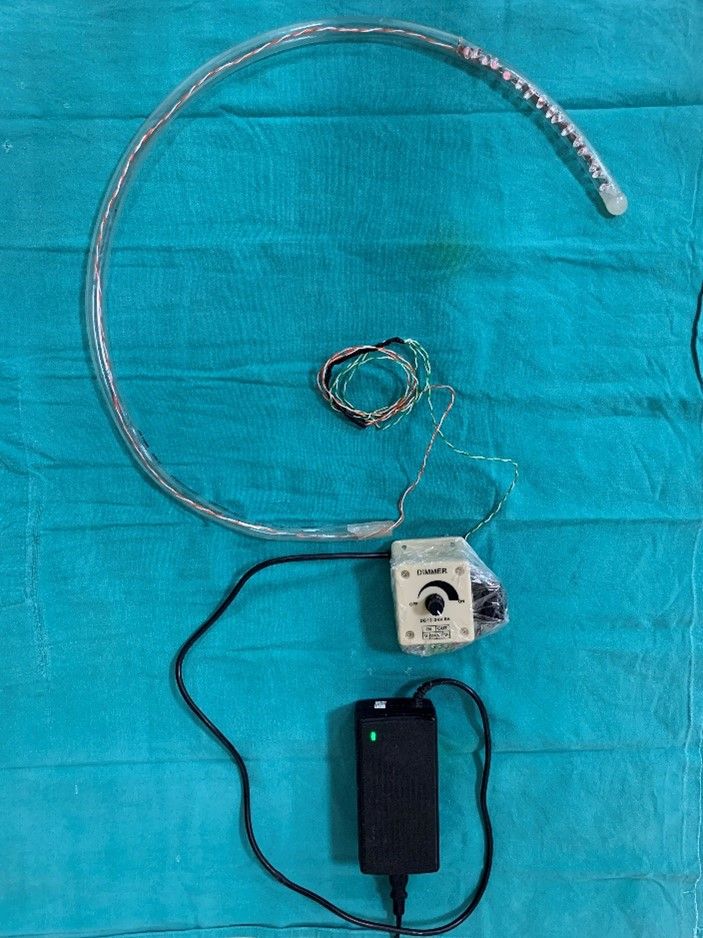
PREPARATION OF THE NEAR INFRA-RED GASTRIC BOUGIE
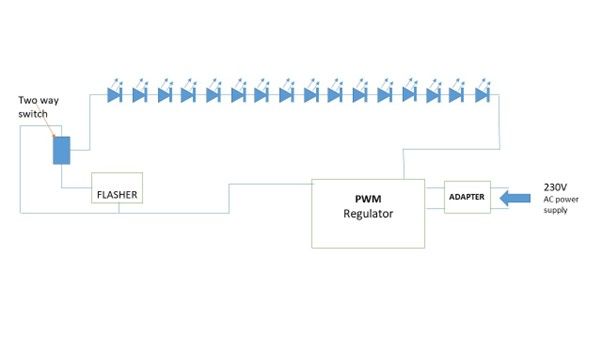
The device preparation requires the following materials, a 39 F transparent tube5 of 75 cm, two rows of eight LEDs (850 nm, 1.5 volts, 100-watt, 30 mA) to cover a distal 20 cm of the tube, an LED blinker, light intensity controller (Fig 1A & 1B). Inside the tube, one row of eight 850 nm LED light bulbs each are connected in series and spirally wrapped around a plastic rod and inserted into the transparent tube. The tube is then sealed at the distal end and only a small opening is made on the wall of tube for suction port. The wires were then connected to a 24-volt PWM (Pulse with Modulation) intensity regulator. A flasher was then connected in series for blinking feature of the device and this was achieved with connecting a two-way switch. This setup was then connected to an adapter and finally to a power source (Figure 2).

Figure 1. A)- In house prepared INFRA – RED GASTRIC BOUGIE.
Courtesy @ World Laparoscopy Hospital, Gurgaon.
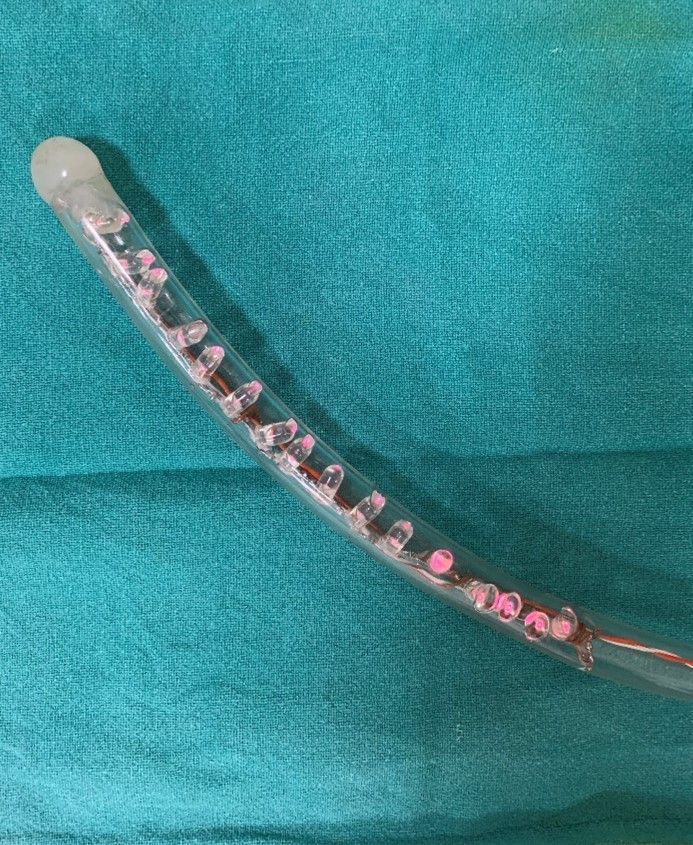

Figure 1. B) – Distal end of gastric bougie with Infra- red LED bulbs.
Courtesy @ World Laparoscopy Hospital, Gurgaon.

Figure 2) Ray diagram showing Infra-red gastric bougie device preparation
WORKING PRINCIPLE OF THE DEVICE
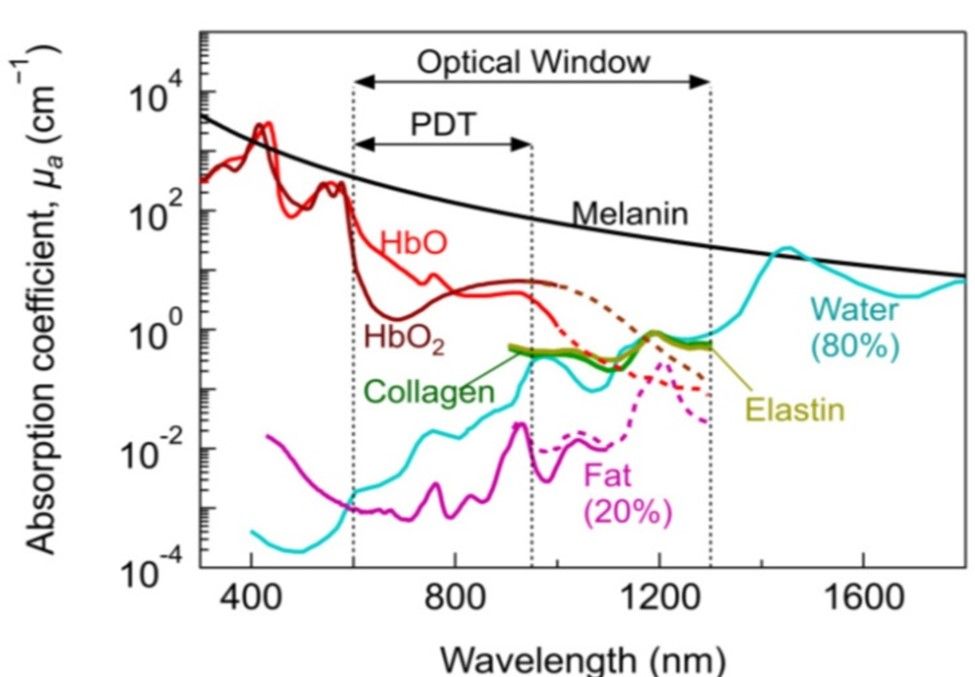
Most of the biological soft tissues have relatively low light absorption property in the visible and NIR spectral regions (600 nm-1300 nm) and is known as a “tissue optical window” or “therapeutic window”6. The fact that the visible light is greatly scattered and absorbed within the depth of just a few hundred microns, this makes near infra-red radiation a good spectral region for performing non-destructive measurements on thick or bulky biological tissue6. The Figure 3 shows absorption co-efficient of various biological tissues at different wavelength spectrum. In our study, 850 nm wavelength emitting LEDs were specifically used as the present-day laparoscopic cameras are designed to capture this wavelength (800- 850 nm) because the cameras are designed to capture the ICG (Indo Cyanin Green) dye at 830 nm. In a dilute aqueous solution, ICG dye has an absorption peak of 780 nm wavelength and an emission peak of 830 nm wavelength7. The normal gastric wall thickness ranges from 2-7 mm in the body and pyloric regions of the stomach respectively8.

Figure 3) - Absorption co-efficient of various biological tissues at different wavelength spectrum.
Courtesy @ Algorri JF et al9.
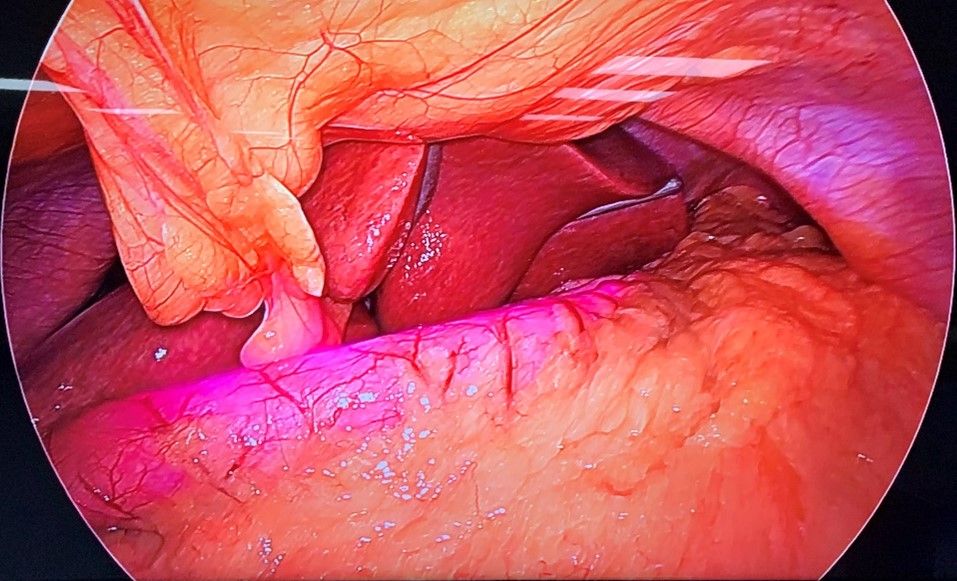
The infra-red light emitted from the diode placed in the gastric bougie penetrates the stomach wall and is captured by the Charged Coupled Device (CCD) attached to the telescope and then visualised on a monitor as a pink fluorescent lit tube with in the stomach lumen and thus acting as a template during various bariatric procedures.
SURGICAL TECHNIQUE
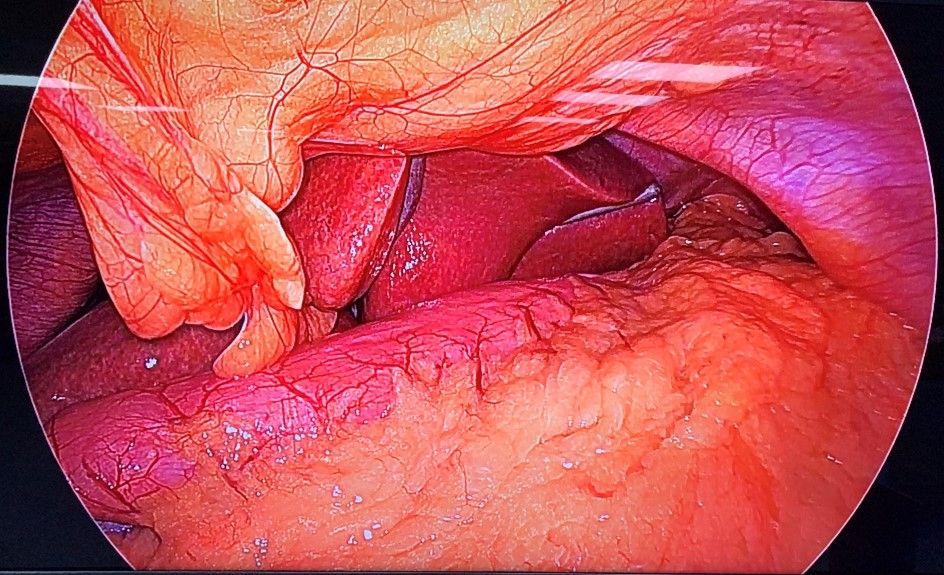
The patient was subjected to general anaesthesia and pneumoperitoneum of 15 mmHg was created. Abdomen was then accessed with 10 mm umbilical port and diagnostic laparoscopy was done. Gastroepiploic gastric branches were then divided starting at 2 cm proximal to the pylorus until the angle of His, dividing the short gastric and posterior fundic vessels after exposing greater curvature10. Infra-red gastric bougie was introduced by the anaesthesiologist into the stomach and the surgeon manipulated the stomach and thus guided the tube into it. The difference in the visualization of the stomach with and without infrared light was noted (Fig 2A & Fig 2B). The gastrectomy was performed over this tube, a maneuver that calibrates the width of the remaining stomach during the resection11. Similarly, its role in gastric plication and in assessing proper closure of the hiatus hernia makes it an important tool during these surgical procedures.

Figure 4. A) - Intra – operative view of stomach with conventional Gastric Bougie.
Courtesy @ World Laparoscopy Hospital, Gurgaon.

Figure 4. B) - Intra – operative view of stomach with Infra-red Gastric Bougie.
Courtesy @ World Laparoscopy Hospital, Gurgaon.
Enabling conventional gastric bougie with infra-red light emitting property easily helped in visually calibrating over the tube and better assessment of the approximate required width during resection. The duration of the surgery was also decreased and procedure was relatively easy to perform.
DISCUSSION
Obesity has almost tripled since 1975 around the world12. As per recent WHO estimates, 2.8 million people die globally per year from complications related to obesity12. Patients with obesity are at major risk for developing cardiovascular disease (CVD), gastrointestinal disorders, type 2 diabetes (T2D), joint and muscular disorders, respiratory problems, and psychological issues, which may significantly affect their daily lives as well as increasing mortality risks13. The Need for increased bariatric procedures is evident with the increase in the prevalence of diabetes worldwide14. Bariatric procedures such as laparoscopic sleeve gastrectomy (LSG), Gastric plication of greater curvature, and others which offer better weight loss results in severe obesity demands continuous research and development to improve the outcome10. LSG involves longitudinal resection of the greater curvature of stomach, starting from the antrum up to the angle of His15. During the longitudinal gastrectomy that “sleeves” the stomach to reduce it to a narrow tube, a gastric bougie is used to obtain a precise calibration and to avoid stenosis of the gastric plasty15. Similarly, the role of bougie in recently popularising gastric plication technique is of paramount importance for gastric calibration, which involves reducing stomach capacity by infolding the greater curvature with sutures. Knowing this we attempted to improve the function of the gastric bougie using infra-red property of light.
In our study, we present to you an easy method of developing a near infra- red gastric bougie (NIR) and its role in the laparoscopic sleeve gastrectomy and comparing it with conventional gastric bougie. Laparoscopic Sleeve Gastrectomy (LSG) has evolved as a standalone procedure for morbid obesity, which involves removing approximately 80% of the stomach, leaving only a sleeve of stomach10. It works by reducing the volume of stomach to create satiety with small amount of food intake16. In our study, a 39 F size tube was used to accommodate the led diode bulbs which can be decreased17. Calibrating the residual stomach for resection is the key in the success of the procedure16. Furthermore, frequent manipulation of the gastric bougie for calibrating residual stomach increases the difficulty of the surgery and thus, increased duration of the procedure. This also increases the risk of injury18. This difficulty led to an idea like use of an infra –red light to visualise the gastric bougie. Knowing this we integrated the above-mentioned principle with conventional gastric bougie and intraoperatively we observed that the near infra-red gastric bougie was easily visualised as a pink fluorescent illuminated tube within the stomach and thus its role as a template during resection of the stomach was enhanced and the frequency of need for manipulating the gastric bougie during the surgery was significantly reduced and thus helped in enhancing the surgical experience.
CONCLUSION
Enhancing a conventional gastric bougie with a near infra-red-light source improves the functionality of the gastric bougie as a template and thus improved surgical experience.
REFERENCES
1. Padwal RS. Characteristics of patients undergoing bariatric surgery in Canada. Obes Res 2005;13(12):2052-4
2. Garofalo F, Pescarus R, Denis R, Atlas H, Garneau P, Philie M, Sayegh K. Laparoscopic Sleeve Gastrectomy: A Radiological Guide to Common Postsurgical Failure. Can AssocRadiol J. 2018 May;69(2):184-196. doi: 10.1016/j.carj.2017.10.004. Epub 2018 Feb 1. PMID: 29395252
3. Kourkoulos M, Giorgakis E, Kokkinos C, et al. Laparoscopic gastric plication for the treatment of morbid obesity: a review. Minim Invasive Surg. 2012;2012:696348. doi:10.1155/2012/696348
4. Tsai, Cheng-Lun& Chen, Ji-Chung & Wang, Wen-Jwu. (2001). Near-infrared Absorption Property of Biological Soft Tissue Constituents. Journal of Medical and Biological Engineering. 21
5. D. L. Wetzel, “Near-infrared reflectance analysis”, Anal. Chem. 55: 1165A-1175A, 1983
6. R. R. Anderson and J. A. Parrish, “The optics of human skin”, J. Invest. Dermatol. 77: 13-19, 1981
7. Yuan B, Chen N, Zhu Q. Emission and absorption properties of indocyanine green in Intralipid solution. J Biomed Opt. 2004;9(3):497-503. doi:10.1117/1.1695411
8. Jeffrey Klein, Emily N. Vinson, Clyde A. Helms, William E. Brant. Brant and Helms' Fundamentals of Diagnostic Radiology. (2018)
9. Algorri JF, Ochoa M, Roldán-Varona P, Rodríguez-Cobo L, López-Higuera JM. Light Technology for Efficient and Effective Photodynamic Therapy: A Critical Review. Cancers. 2021; 13(14):3484
10. Braghetto I, Korn O, Valladares H, Gutiérrez L, Csendes A, Debandi A, Castillo J, Rodríguez A, Burgos AM, Brunet L. Laparoscopic sleeve gastrectomy: surgical technique, indications and clinical results. Obes Surg. 2007 Nov;17(11):1442-50. doi: 10.1007/s11695-008-9421-2
11. Rosenthal R.J., International Sleeve Gastrectomy Expert Panel, Diaz A.A., Arvidsson D., Baker R.S., Basso N., Bellanger D., Boza C., El Mourad H., France M., Gagner M., Galvao-Neto M., Higa K.D., Himpens J., Hutchinson C.M., Jacobs M., Jorgensen J.O., Jossart G., Lakdawala M., Nguyen N.T., Nocca D., Prager G., Pomp A., Ramos A.C., Rosenthal R.J., Shah S., Vix M., Wittgrove A., Zundel N. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of&12,000 cases. Surg. Obes. Relat. Dis. 2012;8(1):8–19
12. Pugliese, G., Liccardi, A., Graziadio, C. et al. Obesity and infectious diseases: pathophysiology and epidemiology of a double pandemic condition. Int J Obes 46, 449–465 (2022). https://doi.org/10.1038/s41366-021-01035-6
13. Fruh SM. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J Am Assoc Nurse Pract. 2017 Oct;29(S1):S3-S14. doi: 10.1002/2327-6924.12510
14. Kaveeshwar SA, Cornwall J. The current state of diabetes mellitus in India. Australas Med J. 2014;7(1):45-48. Published 2014 Jan 31. doi:10.4066/AMJ.2013.1979
15. Iannelli A, Dainese R, Piche T, Facchiano E, Gugenheim J. Laparoscopic sleeve gastrectomy for morbid obesity. World J Gastroenterol. 2008 Feb 14;14(6):821-7. doi: 10.3748/wjg.14.821
16. Aldaqal SM, Al-Amoodi MS (2013) Effect of Bougie Size and Level of Gastric Resection on Weight Loss Post Laparoscopic Sleeve Gastrectomy. J Obes Weight Loss Ther 3: 200
17. Signorini FJ, Verónica G, Marcos M, et al. Iatrogenic injury of the intrathoracic oesophagus with bougie during sleeve gastrectomy. J Minim Access Surg. 2018;14(1):79–82
18. Zhang F, Strain GW, Lei W, Dakin GF, Gagner M, et al. (2011) Changes in lipid profiles in morbidly obese patients after laparoscopic sleeve gastrectomy (LSG). Obes Surg 21: 305-309

.jpeg)
