Page 32 - World Journal of Laparoscopic Surgery
P. 32
Alaa H Ali
Articles that met the following criteria were included in the medication under each two-drug comparison. In some studies,
meta-analysis: counts were calculated from percentages identified in tables or
1. The study was a double-blinded, randomized, controlled figures. Studies with different drug doses within the therapeutic
trial; range. In the study where the patients received crystalloid fluid
12
2. Patients underwent general anesthesia for laparoscopy; (JJ magner) divided the patient into two group the CSL-10
3. Vomiting, nausea, or the use of rescue antiemetic therapy group (n = 70) received compound sodium lactate (CSL) 10 ml
were identified as outcomes; kg–1; the CSL-30 group (n = 70) received CSL 30 ml kg–1. CSL
4. Antiemetic therapy was administered prophylactically, not contains sodium 131 mmol litre–1, potassium 5 mmol litre–1,
just in the treatment of PONV; calcium 2 mmol litre–1, chloride 111 mmol litre–1 and lactate 29
5. At least two drugs (metoclopramide 10 mg, droperidol 20 mmol litre–1. To maintain patient and investigator blinding, intra-
microgram, ondansetron 2 mg, dexamethasone 2 mg IV venous fluid administration was initiated in the preoperative
crystalloid fluid 10 ml/kg and 30 ml/kg) were compared. area.
The meta-analyses were designed to determine the relative
efficacy of ondansetron, droperidol, metoclopramide, RESULT
dexamethasone and IV crystalloid fluid compared with each The details of the articles involving a total of 676 patients
other in reducing the odds of PONV. Separate meta-analyses included in the meta-analyses. The meta-analysis comparing
were performed for the different drug combinations. All patients the efficacy of ondansetron versus metoclopramide included
12
from the included studies were categorized as having 175 patients (Tables 1 and 2). Droperidol versus
13
postoperative vomiting or nausea or using rescue antiemetic metoclopramide analysis included (Table 2). The ondansetron
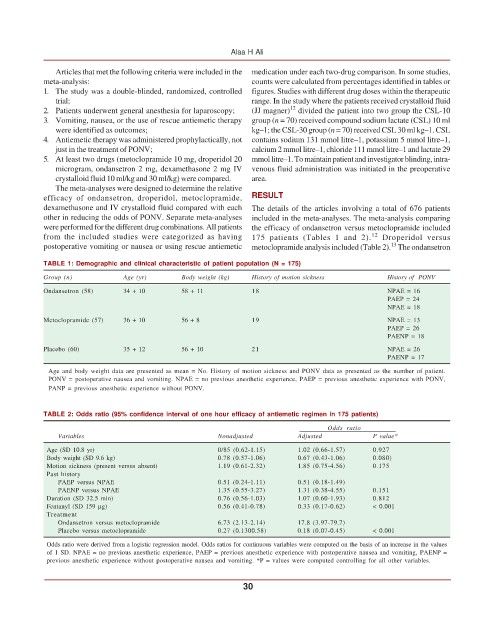
TABLE 1: Demographic and clinical characteristic of patient population (N = 175)
Group (n) Age (yr) Body weight (kg) History of motion sickness History of PONV
Ondansetron (58) 34 + 10 58 + 11 18 NPAE = 16
PAEP = 24
NPAE = 18
Metoclopramide (57) 36 + 10 56 + 8 19 NPAE = 13
PAEP = 26
PAENP = 18
Placebo (60) 35 + 12 56 + 10 21 NPAE = 26
PAENP = 17
Age and body weight data are presented as mean = No. History of motion sickness and PONV data as presented as the number of patient.
PONV = postoperative nausea and vomiting. NPAE = no previous anesthetic experience, PAEP = previous anesthetic experience with PONV,
PANP = previous anesthetic experience without PONV.
TABLE 2: Odds ratio (95% confidence interval of one hour efficacy of antiemetic regimen in 175 patients)
Odds ratio
Variables Nonadjusted Adjusted P value*
Age (SD 10.8 yr) 0/85 (0.62-1.15) 1.02 (0.66-1.57) 0.927
Body weight (SD 9.6 kg) 0.78 (0.57-1.06) 0.67 (0.43-1.06) 0.080)
Motion sickness (present versus absent) 1.19 (0.61-2.32) 1.85 (0.75-4.56) 0.175
Past history
PAEP versus NPAE 0.51 (0.24-1.11) 0.51 (0.18-1.49)
PAENP versus NPAE 1.35 (0.55-3.27) 1.31 (0.38-4.55) 0.151
Duration (SD 32.5 min) 0.76 (0.56-1.03) 1.07 (0.60-1.93) 0.812
Fentanyl (SD 159 μg) 0.56 (0.41-0.78) 0.33 (0.17-0.62) < 0.001
Treatment
Ondansetron versus metoclopramide 6.73 (2.13-2.14) 17.8 (3.97-79.7)
Placebo versus metoclopramide 0.27 (0.1300.58) 0.18 (0.07-0.45) < 0.001
Odds ratio were derived from a logistic regression model. Odds ratios for continuous variables were computed on the basis of an increase in the values
of 1 SD. NPAE = no previous anesthetic experience, PAEP = previous anesthetic experience with postoperative nausea and vomiting, PAENP =
previous anesthetic experience without postoperative nausea and vomiting. *P = values were computed controlling for all other variables.
30