Navigating the Regulatory Landscape of Robotic Surgery Innovations
Introduction
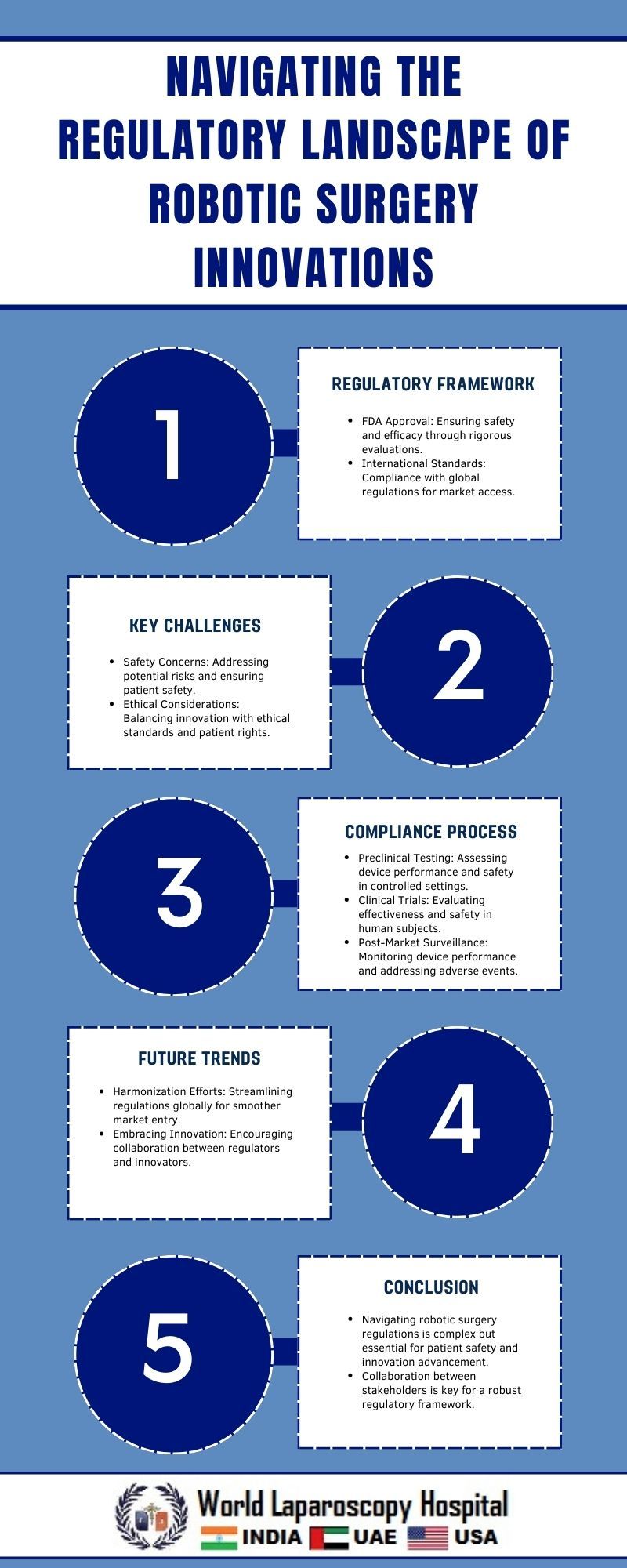
Robotic surgery has emerged as a transformative force in modern healthcare, promising greater precision, less invasive procedures, and improved patient outcomes. However, alongside these advancements come complex regulatory challenges. Navigating the regulatory landscape is essential to ensure the safety, efficacy, and ethical use of robotic surgical innovations. This article explores the regulatory framework surrounding robotic surgery, the challenges it poses, and strategies for navigating these hurdles effectively.
Understanding Robotic Surgery
Robotic surgery involves the use of robotic systems to assist surgeons in performing complex procedures with enhanced precision and control. These systems typically consist of a console where the surgeon sits, controlling robotic arms equipped with surgical instruments, and a high-definition camera providing a magnified, 3D view of the surgical site. Robotic surgery offers several advantages over traditional approaches, including smaller incisions, reduced blood loss, shorter recovery times, and improved surgical outcomes.
The Regulatory Landscape
Regulatory oversight of robotic surgery innovations primarily falls under the jurisdiction of agencies like the Food and Drug Administration (FDA) in the United States and similar regulatory bodies worldwide. These agencies are tasked with evaluating the safety and effectiveness of medical devices, including robotic surgical systems, before they can be marketed and used in clinical practice.
The regulatory pathway for robotic surgical innovations typically involves several stages:
Preclinical Testing:
Before human trials can begin, robotic surgical systems undergo extensive preclinical testing to assess their mechanical reliability, safety features, and compatibility with surgical procedures.
Investigational Device Exemption (IDE) Studies:
If preclinical testing yields promising results, developers may seek IDE approval from regulatory authorities to conduct clinical trials. IDE studies aim to evaluate the safety and efficacy of the robotic system in human subjects under controlled conditions.
Clinical Trials:
Clinical trials are conducted to gather data on the performance of the robotic surgical system in real-world settings. These trials involve careful monitoring of patient outcomes, surgical complications, and long-term effects to determine the system's overall effectiveness and safety.
Regulatory Review:
Following the completion of clinical trials, developers submit a comprehensive application to regulatory agencies for marketing approval. The regulatory review process involves a thorough assessment of the clinical data, manufacturing processes, labeling, and post-market surveillance plans to ensure compliance with regulatory standards.
Challenges in Regulatory Compliance
Despite the potential benefits of robotic surgery, navigating the regulatory landscape poses significant challenges for developers, healthcare providers, and regulatory agencies alike:
Safety Concerns:
Ensuring the safety of patients undergoing robotic surgery is paramount. Technical failures, software glitches, or operator errors can have serious consequences, leading to patient harm or adverse outcomes.
Efficacy and Clinical Outcomes:
Robotic surgical innovations must demonstrate clear benefits over existing treatment modalities to justify their adoption. Generating robust clinical evidence through well-designed trials is essential to establish the efficacy and superiority of these technologies.
Regulatory Uncertainty:
Rapid advancements in robotic surgery often outpace regulatory frameworks, leading to uncertainty regarding the appropriate standards and requirements for market approval. Regulatory agencies must adapt quickly to keep pace with technological innovations while maintaining rigorous oversight.
Cost and Accessibility:
The high cost of robotic surgical systems and associated training can limit their accessibility, particularly in resource-constrained healthcare settings. Balancing cost considerations with patient needs and technological advancements presents a significant challenge for healthcare providers and policymakers.
Strategies for Navigating Regulatory Hurdles
To effectively navigate the regulatory landscape of robotic surgery innovations, stakeholders can employ several strategies:
Early Engagement with Regulatory Agencies:
Developers should proactively engage with regulatory agencies early in the product development process to gain insights into regulatory requirements, anticipate potential challenges, and streamline the regulatory pathway.
Robust Clinical Trial Design:
Designing well-controlled clinical trials with clear endpoints and rigorous methodologies is essential for generating high-quality data on the safety and efficacy of robotic surgical systems. Collaboration with clinical experts and regulatory advisors can help optimize trial designs and ensure compliance with regulatory standards.
Post-Market Surveillance:
Continuous monitoring of robotic surgical systems post-market approval is critical for detecting and addressing any safety concerns or adverse events that may arise during routine clinical use. Establishing robust post-market surveillance mechanisms enables timely reporting and intervention to safeguard patient safety.
Collaboration and Knowledge Sharing:
Collaboration among developers, healthcare providers, regulatory agencies, and patient advocacy groups fosters knowledge sharing and best practices in robotic surgery regulation. By leveraging collective expertise and resources, stakeholders can address regulatory challenges more effectively and promote innovation in healthcare delivery.
Conclusion
Robotic surgery holds immense promise for revolutionizing surgical practice and improving patient outcomes. However, navigating the regulatory landscape presents formidable challenges that require careful consideration and strategic planning. By adopting proactive engagement, rigorous clinical evaluation, and collaborative approaches, stakeholders can navigate regulatory hurdles effectively and ensure the safe and ethical integration of robotic surgical innovations into clinical practice. As technology continues to advance, ongoing dialogue and adaptation of regulatory frameworks will be essential to promote innovation while upholding patient safety and quality of care in robotic surgery.


