Obesity is a major public health concern, affecting millions of people worldwide. It is associated with a range of health complications, including type 2 diabetes, cardiovascular disease, and certain types of cancer. Non-surgical approaches to weight loss, such as diet and exercise, are often recommended as the first-line treatment for obesity. However, these approaches can be difficult to maintain and may not be effective for everyone. In recent years, there has been growing interest in non-surgical solutions for obesity, such as the ORBERA weight loss system. In this essay, we will discuss the ORBERA weight loss system, its approval by the FDA, and its potential benefits for people with obesity.
ORBERA Weight Loss System:
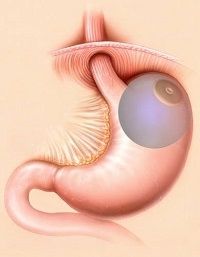
The ORBERA weight loss system is a non-surgical solution for obesity that involves the placement of a soft, silicone balloon in the stomach. The balloon is inflated with a saline solution, which fills up the stomach and creates a feeling of fullness. This reduces the amount of food that a person can eat at one time, leading to a reduction in calorie intake and weight loss.
The ORBERA weight loss system is designed to be used as part of a comprehensive weight loss program that includes diet and exercise counseling. It is intended for people with a BMI of 30 to 40 who have not been able to achieve significant weight loss through diet and exercise alone. The system is not suitable for people with certain medical conditions, such as previous gastrointestinal surgery, esophageal varices, or inflammatory bowel disease.
The ORBERA weight loss system is temporary and is typically removed after six months. During this time, the person is closely monitored by a healthcare provider to ensure that the system is working properly and to provide support and guidance for diet and exercise.
FDA Approval:
The ORBERA weight loss system was developed by Apollo Endosurgery, Inc. and was approved by the U.S. Food and Drug Administration (FDA) in 2015. The approval was based on the results of a clinical trial involving 255 people with a BMI of 30 to 40. The trial found that people who received the ORBERA weight loss system lost an average of 21.8 pounds (9.9 kg), or 10.2% of their body weight, after six months. This was significantly more than the weight loss achieved by people who received a placebo, who lost an average of 6.4 pounds (2.9 kg), or 3.3% of their body weight.
The FDA approval of the ORBERA weight loss system was based on the safety and efficacy of the device. The system was found to be safe and well-tolerated, with no serious adverse events reported. The most common side effects were nausea, vomiting, and abdominal pain, which typically resolved within a few days.
Potential Benefits of ORBERA Weight Loss System:
The ORBERA weight loss system offers several potential benefits for people with obesity. First, it is a non-surgical solution that does not require incisions or anesthesia. This can reduce the risk of complications associated with surgery, such as bleeding, infection, and anesthesia-related complications.
Second, the ORBERA weight loss system is temporary and reversible. Unlike surgical weight loss procedures, such as gastric bypass or sleeve gastrectomy, the ORBERA weight loss system can be removed after six months. This allows for a more flexible approach to weight loss, as the person can choose to continue with non-surgical approaches or pursue surgical options if necessary.
Third, the ORBERA weight loss system is designed to be used as part of a comprehensive weight loss program that includes diet and exercise counseling. This can provide people with the support and guidance they need to make sustainable lifestyle changes and achieve long-term weight loss .
Fourth, the ORBERA weight loss system has been shown to be effective in promoting weight loss. The clinical trial that led to the FDA approval of the system found that people who received the ORBERA weight loss system lost an average of 21.8 pounds (9.9 kg), or 10.2% of their body weight, after six months. This was significantly more than the weight loss achieved by people who received a placebo.
Fifth, the ORBERA weight loss system may improve obesity-related health complications. Obesity is associated with a range of health complications, such as type 2 diabetes, cardiovascular disease, and certain types of cancer. Weight loss can improve these complications, and the ORBERA weight loss system has been shown to be effective in reducing the risk of type 2 diabetes and improving metabolic parameters such as blood pressure, cholesterol, and blood glucose levels.
Sixth, the ORBERA weight loss system may improve quality of life. Obesity can have a negative impact on a person's quality of life, leading to social stigmatization, decreased mobility, and reduced self-esteem. Weight loss can improve these aspects of quality of life, and the ORBERA weight loss system has been shown to be effective in improving quality of life measures such as self-esteem, physical function, and mental health.
Limitations of ORBERA Weight Loss System:
Despite its many potential benefits, the ORBERA weight loss system has some limitations that must be considered. First, the system is not suitable for everyone. It is intended for people with a BMI of 30 to 40 who have not been able to achieve significant weight loss through diet and exercise alone. It is not suitable for people with certain medical conditions, such as previous gastrointestinal surgery, esophageal varices, or inflammatory bowel disease.
Second, the ORBERA weight loss system can cause side effects. The most common side effects are nausea, vomiting, and abdominal pain, which typically resolve within a few days. However, some people may experience more serious side effects, such as balloon rupture or migration, which can require surgical intervention.
Third, the ORBERA weight loss system is not a permanent solution to obesity. It is designed to be used as part of a comprehensive weight loss program that includes diet and exercise counseling. Once the system is removed after six months, the person will need to continue with lifestyle changes to maintain weight loss.
Fourth, the ORBERA weight loss system can be expensive. The cost of the system can vary depending on the location and the healthcare provider. Insurance coverage for the system may also vary.
Conclusion:
The FDA approval of the ORBERA weight loss system provides a non-surgical solution for people with obesity who have not been able to achieve significant weight loss through diet and exercise alone. The system offers several potential benefits, such as being non-surgical, temporary, and effective in promoting weight loss and improving health outcomes. However, it is not suitable for everyone and can cause side effects. The ORBERA weight loss system should be used as part of a comprehensive weight loss program that includes diet and exercise counseling. Further research is needed to determine the long-term outcomes and cost-effectiveness of the system.