Laparoscopic Tubal Surgery
Tubal diseases are one of the frequent causes of infertility. The most common predisposing factor is a pelvic inflammatory disease. Distal tubal obstruction has been managed previously by open surgery using a microsurgical technique. The pregnancy rate after reconstructive surgery is 20 to 30 percent two years postoperatively. Laparoscopy for tubal infertility has been a significant factor in reducing—costs, hospitalization, and recuperation. Recently, in women with severe tubal damage, in vitro fertilization (IVF) offers a better chance for term pregnancy (72.3%) compared to reconstructive surgery (27.3%). Fimbrioplasty and lysis of peritubular and periovarian adhesions have been associated with good pregnancy rates. In these patients, IVF is appropriate when pregnancy is not achieved postoperatively after a few years.
Laparoscopic Tubal Anatomy
The fallopian tubes arise from the superior portion of the uterus just above the attachment points of the round ligament. Laparoscopically, the round ligaments overhang the fallopian tube because of uterine manipulation and can be easily mistaken for them. The fallopian tubes towards its lateral end encircle the ovaries partially with their fimbriated ends. From anterior to posterior, the following important tubular structures are found crossing the brim of the true pelvis; the round ligament of the uterus, the infundibulopelvic ligament, which contains the gonadal vessels and the ureter. The ovaries and fallopian tubes are found between the round ligament and the infundibulopelvic ligament. The ovarian ligaments run from the ovaries to the lateral border of the uterus. The ovary is attached to the pelvic sidewall with infundibulopelvic ligament, which carries the ovarian artery. One of the common mistakes is the injury of the ureter during dissection of the infundibulopelvic ligament. If the uterus deviates to the contralateral side with the help of uterine manipulator infundibulopelvic ligament is spread out and a pelvic sidewall triangle is created. The base of this triangle is the round ligament, the medial side is the infundibulopelvic ligament, and the lateral side is the external iliac artery. The apex of this triangle is the point at which the infundibulopelvic ligament crosses the external iliac artery.
The ureters enter the pelvis in close proximity to the female pelvic organ and are at risk for injury during laparoscopic surgery of these organs. As the ureter course medially over the bifurcation of the iliac vessels, they pass obliquely under the ovarian vessels and then run in close proximity to the uterine artery.

Tubal anatomy
Patient Position
The patient should be in steep Trendelenburg and lithotomy position. One assistant should remain between the legs of the patient to do uterine manipulation whenever required.
Port Position
The port position should be in accordance with the baseball diamond concept. If the left side of the tube has to be operated, one port should be in the right iliac fossa and another below left hypochondrium.
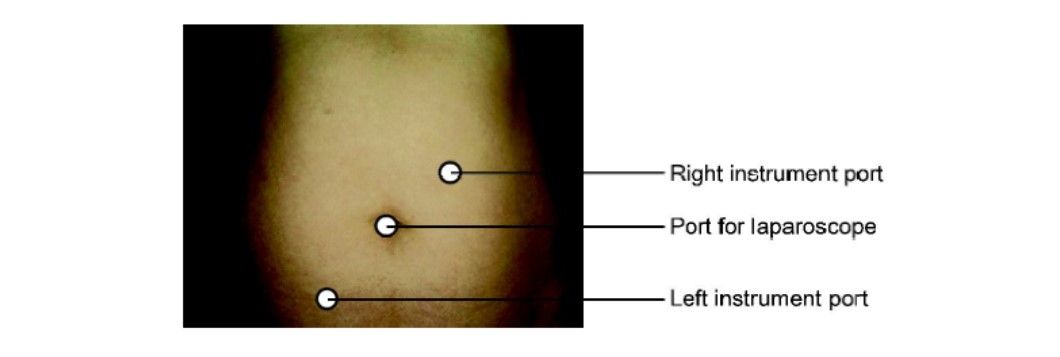
Port position tubal surgery left side
Operative Procedure
Management of Acute PID
Pelvic inflammatory disease (PID) usually results from sexually transmitted diseases caused by Chlamydia or Gonococcus infection, an intrauterine device (IUD), postpartum endometritis, or hysteroscopy at the time of endometrial infection.
The pelvic inflammatory disease has four primary sequelae:
1. Infertility
2. Ectopic pregnancy
3. Chronic pelvic pain
4. Recurrent upper UTI.
One of the worst outcomes of PID is adhesions of the reproductive organ leading to infertility and pain. The degree of tubal damage and pelvic adhesions often depends on the severity of the infection, the number of PID episodes, and etiology. Severe peritonitis is associated with a 17 percent risk of infertility compared to 3 percent for a mild infection. With each successive episode of PID, the risk of infertility doubles. Despite its typically mild presentation, chlamydial PID results in a three-fold increase in infertility compared to gonococcal PID. The risk of ectopic pregnancy is 6 to 10 times higher in women who have had PID. In addition, chronic pelvic pain has been shown to occur in 15 to 18 percent of patients after PID, usually because of adhesions. Up to 20 to 25 percent of patients will have at least one recurrent infection because damaged fallopian tubes are more susceptible to infection.
Laparoscopy is being used increasingly in patients suspected of having PID to make a precise diagnosis and thereby avoid the potential sequelae. Prompt surgical confirmation of the diagnosis is possible with laparoscopy. A tubo-ovarian abscess (TOA) can be drained, reducing the risk of serious morbidity associated with rupture. The clinical diagnosis of PID is difficult because of the wide variation in symptoms and signs. Many women with PID report subtle, vague, or mild symptoms that are not specific, such as dyspareunia, postcoital spotting, or abnormal uterine bleeding. In these situations, a bimanual examination may demonstrate cervical mobility or adnexal tenderness.
A tubo-ovarian abscess is severe sequelae and occurs in as many as 34 percents of patients hospitalized with PID. Symptomatic or subclinical infections can progress rapidly into a TOA. These abscesses can rupture, resulting in severe peritonitis.
At present, surgical intervention is used only to treat a tubo-ovarian mass when medical management is ineffective. The laparoscopic procedure for managing pelvic abscesses has been described by several authors. Once TOA is diagnosed laparoscopically, two 5 mm trocars are placed in both the flanks according to the baseball diamond concept through which a suction -irrigator probe and grasping forceps are inserted. The pelvis, upper abdomen, and pelvic gutters should be examined for free or loculated purulent material and the course of both ureters should be identified. The purulent fluid is aspirated from the pelvis, and cultures are taken from the aspirated fluid and the inflammatory exudates. If necessary, the suction-irrigator is used to mobilize the omentum, small bowel, rectosigmoid. Tubo-ovarian adhesions should be recognized until the abscess cavity is localized. After the abscess cavity is drained, the suction— irrigator is used to separate the bowel and omentum completely from the reproductive organs. Chromotubation is not indicated in the case of PID because edema in the interstitial tissue of the tube occludes the lumen.
At the end of the procedure, the whole peritoneal cavity is irrigated with normal saline until the effluent is clear. Between 300 and 400 ml of irrigation fluid are left in the pelvis to separate these organs during the early healing phase. Adhesiolysis is technically difficult and associated with a high risk of complications. Hydrodissection decreases the potential for intestinal or ureteral injury; the laser and electrosurgery should be used sparingly.
Tubal diseases are one of the frequent causes of infertility. The most common predisposing factor is a pelvic inflammatory disease. Distal tubal obstruction has been managed previously by open surgery using a microsurgical technique. The pregnancy rate after reconstructive surgery is 20 to 30 percent two years postoperatively. Laparoscopy for tubal infertility has been a significant factor in reducing—costs, hospitalization, and recuperation. Recently, in women with severe tubal damage, in vitro fertilization (IVF) offers a better chance for term pregnancy (72.3%) compared to reconstructive surgery (27.3%). Fimbrioplasty and lysis of peritubular and periovarian adhesions have been associated with good pregnancy rates. In these patients, IVF is appropriate when pregnancy is not achieved postoperatively after a few years.
Laparoscopic Tubal Anatomy
The fallopian tubes arise from the superior portion of the uterus just above the attachment points of the round ligament. Laparoscopically, the round ligaments overhang the fallopian tube because of uterine manipulation and can be easily mistaken for them. The fallopian tubes towards its lateral end encircle the ovaries partially with their fimbriated ends. From anterior to posterior, the following important tubular structures are found crossing the brim of the true pelvis; the round ligament of the uterus, the infundibulopelvic ligament, which contains the gonadal vessels and the ureter. The ovaries and fallopian tubes are found between the round ligament and the infundibulopelvic ligament. The ovarian ligaments run from the ovaries to the lateral border of the uterus. The ovary is attached to the pelvic sidewall with infundibulopelvic ligament, which carries the ovarian artery. One of the common mistakes is the injury of the ureter during dissection of the infundibulopelvic ligament. If the uterus deviates to the contralateral side with the help of uterine manipulator infundibulopelvic ligament is spread out and a pelvic sidewall triangle is created. The base of this triangle is the round ligament, the medial side is the infundibulopelvic ligament, and the lateral side is the external iliac artery. The apex of this triangle is the point at which the infundibulopelvic ligament crosses the external iliac artery.
The ureters enter the pelvis in close proximity to the female pelvic organ and are at risk for injury during laparoscopic surgery of these organs. As the ureter course medially over the bifurcation of the iliac vessels, they pass obliquely under the ovarian vessels and then run in close proximity to the uterine artery.

Tubal anatomy
Patient Position
The patient should be in steep Trendelenburg and lithotomy position. One assistant should remain between the legs of the patient to do uterine manipulation whenever required.
Port Position
The port position should be in accordance with the baseball diamond concept. If the left side of the tube has to be operated, one port should be in the right iliac fossa and another below left hypochondrium.

Port position tubal surgery left side
Operative Procedure
Management of Acute PID
Pelvic inflammatory disease (PID) usually results from sexually transmitted diseases caused by Chlamydia or Gonococcus infection, an intrauterine device (IUD), postpartum endometritis, or hysteroscopy at the time of endometrial infection.
The pelvic inflammatory disease has four primary sequelae:
1. Infertility
2. Ectopic pregnancy
3. Chronic pelvic pain
4. Recurrent upper UTI.
One of the worst outcomes of PID is adhesions of the reproductive organ leading to infertility and pain. The degree of tubal damage and pelvic adhesions often depends on the severity of the infection, the number of PID episodes, and etiology. Severe peritonitis is associated with a 17 percent risk of infertility compared to 3 percent for a mild infection. With each successive episode of PID, the risk of infertility doubles. Despite its typically mild presentation, chlamydial PID results in a three-fold increase in infertility compared to gonococcal PID. The risk of ectopic pregnancy is 6 to 10 times higher in women who have had PID. In addition, chronic pelvic pain has been shown to occur in 15 to 18 percent of patients after PID, usually because of adhesions. Up to 20 to 25 percent of patients will have at least one recurrent infection because damaged fallopian tubes are more susceptible to infection.
Laparoscopy is being used increasingly in patients suspected of having PID to make a precise diagnosis and thereby avoid the potential sequelae. Prompt surgical confirmation of the diagnosis is possible with laparoscopy. A tubo-ovarian abscess (TOA) can be drained, reducing the risk of serious morbidity associated with rupture. The clinical diagnosis of PID is difficult because of the wide variation in symptoms and signs. Many women with PID report subtle, vague, or mild symptoms that are not specific, such as dyspareunia, postcoital spotting, or abnormal uterine bleeding. In these situations, a bimanual examination may demonstrate cervical mobility or adnexal tenderness.
A tubo-ovarian abscess is severe sequelae and occurs in as many as 34 percents of patients hospitalized with PID. Symptomatic or subclinical infections can progress rapidly into a TOA. These abscesses can rupture, resulting in severe peritonitis.
At present, surgical intervention is used only to treat a tubo-ovarian mass when medical management is ineffective. The laparoscopic procedure for managing pelvic abscesses has been described by several authors. Once TOA is diagnosed laparoscopically, two 5 mm trocars are placed in both the flanks according to the baseball diamond concept through which a suction -irrigator probe and grasping forceps are inserted. The pelvis, upper abdomen, and pelvic gutters should be examined for free or loculated purulent material and the course of both ureters should be identified. The purulent fluid is aspirated from the pelvis, and cultures are taken from the aspirated fluid and the inflammatory exudates. If necessary, the suction-irrigator is used to mobilize the omentum, small bowel, rectosigmoid. Tubo-ovarian adhesions should be recognized until the abscess cavity is localized. After the abscess cavity is drained, the suction— irrigator is used to separate the bowel and omentum completely from the reproductive organs. Chromotubation is not indicated in the case of PID because edema in the interstitial tissue of the tube occludes the lumen.
At the end of the procedure, the whole peritoneal cavity is irrigated with normal saline until the effluent is clear. Between 300 and 400 ml of irrigation fluid are left in the pelvis to separate these organs during the early healing phase. Adhesiolysis is technically difficult and associated with a high risk of complications. Hydrodissection decreases the potential for intestinal or ureteral injury; the laser and electrosurgery should be used sparingly.





