Laparoscopic Repair of Ventral Hernia
Introduction
Ventral hernias refer to fascial defects of the anterolateral abdominal wall through which intermittent or continuous protrusion of abdominal tissue or organs may occur. They are either congenital or acquired. In adults, more than 80 percent of ventral hernias result from previous surgery hence the term incisional hernias. They have been reported to occur after 0 to 26 percent of abdominal procedures. Although these hernias mostly become clinically manifest between 2 to 5 years after surgery, studies have shown that the process starts within the first postoperative month. They are said to occur as a result of a biomechanical failure of the acute fascial wound coupled with clinically relevant impediments to acute tissue repair and normal support function of the abdominal wall.
Historically, incisional hernias have been repaired with either primary suture techniques or placement of a variety of prosthetic materials. Before the 1960s, most ventral hernias were repaired primarily with suture and a few with metallic meshes. Even with some modifications, recurrence rates with the primary suture repair ranged from 24 to 54 percent. The introduction of polypropylene mesh repair by Usher in 1958 opened a new era of tension-free herniorrhaphy. Recurrence rates with prosthetic mesh decreased to 10 to 20 percent. Subsequently, it was realized that the placement and fixation of the mesh were more crucial in determining the outcome of the repair. The placement of the mesh in the preperitoneal, retro muscular position with a wide overlap of at least 5 cm over the hernia defect in all directions was introduced in the late 1980s. The refinement of this method decreased the recurrence rates to as low as 3.5 percent making it to be declared the standard of care of ventral hernias. However, implantation of the mesh by open techniques requires a wide dissection of soft tissue contributing to an increase in wound infection and wound-related complications.
Ventral hernia results from a weakness in the musculoaponeurotic layer of the anterior abdominal wall. This type of hernia has the root of development during the period of development like; omphalocele, gastroschisis, and congenital umbilical hernia. Recently, the ventral hernias are reported more due to the iatrogenic factor. Even after laparoscopic surgery, if the 10 mm port is not repaired properly there is always a chance of ventral hernia (Incisional hernia) development. Obviously the initial closure is the most important since faulty technique will universally lead to the development of herniation. There are other associated co-morbid conditions, which may encourage the creation of incisional herniation. These include intra-abdominal or wound infection, morbid obesity, steroid use, previous use of the incision, hematoma formation, and respiratory compromise with an increased cough. Other factors include the duration of the operation, crossing incisions, ineffective wound drainage, and excessive wound tension. Two other important variables include nutritional aspects as well as the presence of cancer which overall reduces the ability for wound healing and collagen deposition in the wound.
The repair of incisional and ventral hernias continues to be a surgical challenge. Reports published in the medical literature indicate 3 to 13 percent of laparotomy patients develop incisional hernias. Moreover, clinical studies indicate that the traditional, or open, a technique to repair large abdominal wall defects is associated with recurrence rates ranging from 25 to 49 percent.
Among the non-iatrogenic ventral hernias, divarication of rectus abdominis, umbilical, para-umbilical, Spigelian, and epigastric are more common. In 1992, a successful series of laparoscopic incisional hernia repairs were reported in the medical literature. Since then, the technique has been refined and has grown in acceptance within the surgical community.
The laparoscopic technique for ventral hernia repair involves the placement of a tension-free prosthetic bridge across the musculofacial defect rather than attempting to approximate the edge of the defect. The hernia defect is covered by an appropriate size of mesh once the content of the sac is reduced. Most of the time sac content is omentum. Sometime omentum has adhered so tightly that electrosurgical dissection with the help of bipolar is essential. Recently, a much newer type of mesh is available in which PTFE and polypropylene are more popular. There was always a fear of bowel adhesion and fistulization with the use of polypropylene mesh but the clinical evidence of thousands of surgery has suggested that the omental adhesion is expected but bowel adhesion is not common and intraperitoneal placement of polypropylene mesh is quite safe. Almost all types of ventral hernia can be repaired by minimal access surgical approach. Hernias like multiple defects (Swiss cheese hernias) are greatly benefited by this approach as all defects get directly visualized and appropriately covered by single mesh. Contraindication of laparoscopic repair of ventral hernia is a very large hernia with a huge protrusion of skin which is thin enough. Skinfold is necessary to correct by abdominoplasty. Dense intra-abdominal adhesions are also a relative contraindication of laparoscopic repair of ventral hernia.
Laparoscopic Anatomy
A ventral hernia develops due to the structural weakness of the abdominal wall. The muscle and fascia that span the space between the costal margin superiorly, the spine and muscles of the back posteriorly, and the pelvis inferiorly support the abdominal wall by giving strength. The parietal peritoneum in ventral hernia extends into the defect to form the sac. Adhesions to adjacent viscera must be divided to define the defect.
Operative Procedure
The patient should be clearly informed that laparoscopic repairs will not help cosmetically if the skin is lax and hanging loosely. Bowel preparation is a good practice to have more room inside the abdominal cavity to handle the instrument. After anesthesia, a nasogastric tube is a must to deflate the stomach completely because in most cases access should be through left hypochondria. Splenohepatomegaly is an absolute contraindication of the access through left hypochondrium. The patient is placed in a supine position without any tilt of the operation table so that bowel is distributed evenly.
Position of Surgical Team
The surgeon stands left to the patient with a camera operator on his left or right side depending upon the location of the ventral hernia. If a ventral hernia is below the umbilicus, the camera operator stands right to the patient. If the defect is above the umbilicus, the camera operator should stand left to the patient. The monitor should be placed opposite to the surgeon and the instrument trolley should be towards the leg of the patient.

Operating room set up in ventral hernia repair
Port Position
The technique of laparoscopic repair of ventral hernia is quite simple. The first pneumoperitoneum is created at a site away from the defect. Three port techniques are used for laparoscopic repair of ventral hernia. The first step in performing laparoscopic repair of ventral hernia is gaining access to the free peritoneal cavity. A site distant from any prior incision and the hernia defect is chosen. Typically this is in the right upper quadrant (RUQ) or left upper quadrant (LUQ). The absence of incisions in these locations does not necessarily guarantee the absence of adhesions to viscera. While many approaches for access to the peritoneal cavity have been described, including blind insufflation and specialty trocars, open access in the fashion of Hasson is by far the safest alternative.
Once the pneumoperitoneum is created all other port is placed according to the baseball diamond concept. The most preferred site of access is the left hypochondrium in the most midline and lower abdominal defects.
First access should be preferably through left hypochondria if the Veress needle technique is used and then two other ports should be made so that a proper triangle is formed. The distance between the two ports should not be less than 5 cm. The telescope will first enter through the left hypochondriac port but once dissection starts the telescope will come in the middle so that the angle between two working port will become 60°. The 10 mm 30° telescope is better to view the anterior abdominal wall. The ventral hernia repair can be performed by two techniques. The first technique is the intraperitoneal or Onlay mesh technique in which mesh is placed without dissecting peritoneum. This is also called the Onlay method. All content of the sac is reduced and any adhesion (if present) is cleared. An appropriate size of the mesh is then inserted.
After free access to the peritoneal cavity is obtained. This also represents the greatest risk to the patient. The difficulty of adhesiolysis is unpredictable, although the presence of polypropylene mesh should be a red flag indicating the potential for the presence of dense and difficult to dissect adhesions, often involving the bowel. All maneuvers performed as part of the lysis of adhesions must be done under direct vision. This is best carried out by sharp dissection utilizing bimanual palpation of the anterior abdominal wall, placing the adhesions under variable degrees of tension. There is significant risk in extensive blunt dissection, as the bowel may be fixed at several points placing it at risk for unrecognized perforation with the tip of dissecting instruments. In spite of the enthusiasm for different energy sources, these are best avoided. As in open cases, dissection should be carried out at the avascular junction of the adhesions and the anterior abdominal wall. Ligating clips or the limited application of an energy source can be used when significant bleeding or vessels are encountered. In the majority of cases, even this is unnecessary. The risk of monopolar cautery is well known, but there is also a risk of thermal injury by direct contact with ultrasonic or radiofrequency dissection instruments. This is particularly true in the poorly visualized area behind adhesions.

Bipolar can be used safely in case of adhesion with omentum
If omentum adheres to the anterior abdominal wall it should be dissected after applying bipolar or extracorporeal knot. It is critical that all adhesions to the anterior abdominal wall be released to allow adequate patch placement and fixation. Once adhesiolysis has been completed, the exact extent of the defect can be directly evaluated. The defects are carefully drawn onto the skin of the anterior abdominal. In the case of multiple defects, the area drawn should include all of the defects. We have progressed to repairing the entire area of a previous incision as opposed to simply repairing a single defect. There have been a number of patients who have presented later in follow-up and are discovered to have a new hernia, outside the area of the previous repair. In open surgery, these may have simply been considered recurrences. If there is any difficulty in delineating the margins of the defect, a spinal needle can be passed perpendicular to the anterior abdominal wall and through the margins of the defect.
The selection of the size of the mesh is important to prevent recurrence of hernia and it should be sufficiently big so that approximately 4 cm healthy margin of defect of hernia should be covered all around. Recently new hybrid mesh has been introduced with absorbable material on one side and unabsorbable proline on the other. These meshes are better than proline because adhesion is less likely to develop due to absorbable material towards the bowel.
The mesh is fixed along margins and around the ring of defect of rectus to ensure a close approximation of mesh to the abdominal wall. Care should be taken that mesh should not be corrugated and it should be in proper contact with the anterior abdominal wall. Tacker, Protack, or Endo anchor can be used to fix the mesh. After fixing the mesh greater omentum is speeded like an apron in between the bowel and mesh. Some adhesion of mesh with omentum is always expected in this technique. If a patient is early mobilized and a newer generation of mesh is used the long-term complication of adhesion is very less with this technique.
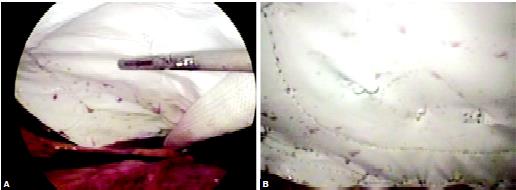
Fixation of the mesh can be accomplished with endoanchor
Loosely held mesh hanging through the anterior abdominal wall will definitely increase the chance of adhesion with bowel. Tacker should be used to fix the mesh in position. Recently, a technique of using proline suture to fix the mesh with the anterior abdominal wall is used with the help of a suture passer or looping technique with the help of a Veress needles cannula. The main idea of this method is to reduce the cost of surgery, but there is an increased chance of infection and adhesion with this method. We also lack any long-term randomized controlled trial to prove the outcome of this external suture technique to fix the mesh in ventral hernia repair.
Choice of Mesh in Ventral Hernia
Synthetic Materials
A variety of synthetic polymeric meshes were developed in the second half of the 20th century and revolutionized hernia repair. With these meshes, abdominal wall defects could be repaired without undue tension on the sutured tissue, decreasing the high recurrence rates of abdominal wall hernia repair. Sir Francis Usher introduced woven monofilament polypropylene mesh in 1958. It was modified to a knitted mesh in 1962 so that the mesh would not unravel when it was cut. Polypropylene mesh gained widespread popularity over the next 30 years and several types of polypropylene mesh are commercially available today. The polyester mesh was also introduced in the 1950s in Europe. Rives and Stoppa employed polyester mesh in their landmark article describing a preperitoneal technique of ventral hernia repair in 1989. The technique described by Rives and Stoppa has become the standard by which all abdominal wall incisional hernia repairs are measured. Polypropylene mesh and polyester mesh revolutionized abdominal wall repair because the meshes did not deteriorate with age, were pliable, and would stretch, allowing for more even load distribution. Nevertheless, the large interstices in polypropylene and polyester mesh promoted adhesion formation when the mesh came into contact with the visceral abdominal cavity. Reported complications included small bowel obstruction, erosion, and fistulization. Expanded polytetrafluoroethylene (ePTFE), initially used as a vascular prosthesis, was adapted for abdominal wall incisional hernia repair in 1983 by WL Gore and Associates and modified several times in the 1990s. Unlike the polypropylene and polyester meshes that preceded it, ePTFE is microporous and select products are uniquely designed with pores measuring 3 microns on the visceral side facing the abdominal cavity and 22 microns facing the abdominal wall. This design promotes fibroblastic and vascular ingrowth from the abdominal wall 22-micron side but inhibits tissue attachment to the material on the 3-micron side when exposed to the intraabdominal cavity. There are no reports of fistulization or small bowel obstructions due to adhesions from ePTFE material.
Synthetic meshes, made of materials such as ePTFE (expanded polytetrafluoroethylene) and polypropylene is used most of the time for repair of the hernia. The repair process for these materials is based on scar formation in and around the mesh. The advantage of using these materials is that they generally do not react with human tissue. They are strong and do not tear easily, are readily available, inexpensive, and have a long history of being used for soft tissue replacements.
However, the use of synthetic materials is not without problems. As a foreign material, the body may react to its presence by growing around it (encapsulation) in an attempt to exclude it from the body. In the process, tissue forms a capsule of rigid, fibrous scar tissue around the synthetic material. The rigid capsule could affect the function and the aesthetic outcome of the repair. Furthermore, foreign bodies such as synthetic materials increase the risk of infection when implanted in the body. As part of the foreign body response, the repair site may be subjected to inflammation, infection, and pain.
Surgisis
It is porcine intestinal submucosa and specifically designed as a surgical graft for hernia and abdominal body wall repair. Surgisis gold combines strength with flexibility in a naturally occurring graft material that allows for hernia repair without the need for a permanent synthetic prosthesis. Surgisis® Gold™ supports the surgical site while the body's natural healing process replaces the graft with new host tissue. It is collagen biomatrix, naturally occurring and acellular with 18 months shelf life.
Alloderm
It is a biological dermal matrix from processed donated human tissue. AlloDerm is processed from donated human skin. The tissue goes through a cell removal process while retaining the important biochemical and structural components. AlloDerm is, thus, acellular human tissue. Since AlloDerm is derived from human tissue, there may be a concern that it might harbor disease-carrying viruses. Tissue donors are screened and tested for transmissible diseases including HIV, hepatitis, and syphilis before tissue processing. AlloDerm has been utilized in more than 7,50,000 implants and grafts to date, without any reported incidence of viral disease transmission to a patient. AlloDerm repairs damaged tissue by providing a foundation for new tissue regeneration. The skin components preserved in AlloDerm contain the information that will help your own tissue to grow into the AlloDerm after placement at the repair site.
Proceed
Soft PPM covered with PDS and oxidized regenerated cellulose fabric.
The Second Technique
Preperitoneal Repair of Ventral Hernia
This technique is also called inlay technique of laparoscopic ventral hernia repair. The peritoneum is incised and preperitoneal space is created in this technique. The sac of the hernia if excised nicely. It can give some extra flap of peritoneum for the successful overlapping of both the edges. In this technique margin of defect is sutured intracorporeally to decrease the gap. Pressing the abdominal wall on both the side from outside will help to obliterate the space in this hernia surgery. Either intracorporeal stitches or external mattress sutures can be used to fix the mesh to the musculofacial defect. Once the mesh is fixed, the peritoneum is sutured using vicryl to cover the mesh. This method of laparoscopic ventral hernia repair is the same as that of open surgery and it is supposed that the formation of adhesion is less.
Complications
• Bowel adhesion
• Fistulization
• Nerve injury
• Vascular injury.
Multiple studies have documented that open hernioplasty has significant morbidity. Leber reported a 27 percent long- term complication rate with the open repair; among them being infection, hematoma, and seroma, chronic sinus tract formation, mesh extrusion, fistula formation as well as soft tissue problems such as a non-healing wound. White reported 34 percent of 250 open ventral hernia repairs had wound-related complications. The complications of open repair mainly relate to the type of mesh that is most commonly used (polypropylene and polyester meshes). In addition, the wide dissection of soft tissue that is required for a Stoppa type retro-rectus repair or a Chevrel type anterior repair leads to the many wound-related problems. Some patients will develop a fluid collection, which is commonly called a seroma, between the mesh and the abdominal wall. Many of these are not apparent to the patient or the surgeon but some are evident and can be bothersome to the patient. Complications from these seromas were sometimes reported in many studies. Most surgeons do not aspirate these fluid collections for fear of infecting the prosthetic. However, the author has freely aspirated the seromas if they are large or if they are bothersome to the patient. The author has never seen an infection of the prosthetic from aspiration of these fluid collections if full aseptic precaution is taken.
Probably the most dreaded complication that has been seen is bowel injury. Enterotomy is a well-documented complication and commonly occurs and can be readily visualized and handled through an incision. Laparoscopy presents a whole new situation with respect to enterotomy. Prevention is the first line of defense. The lysis of adhesions is well visualized due to the magnification and high-intensity light source inherent in the laparoscopic technique. It is very important that energy sources be used very sparingly if at all during lysis of adhesions. If a surgeon enters the proper planes, there is very little bleeding and thus a low need for energy sources. Inappropriate use of energy sources is a common cause of unrecognized enterotomy. Monopolar cautery has the problem of current spread, and it is easy to coagulate one area and see the current spread to the adjacent area instantaneously. For this reason, monopolar cautery should not be used adjacent to the bowel. The ultrasonic or radiofrequency dissection instruments are "sold" with the supposed advantage that there is minimal thermal spread, unlike monopolar cautery. Although this may be true, the tip remains very hot and any touching of viscera can cause a burn that may not be apparent during the operation. It is only after several hours, either that night or the next day, when the tissue sloughs, that the enterotomy presents itself. We do not recommend the use of ultrasonic or radiofrequency dissection instruments for this reason. The most important thing to remember is that if lysis of adhesions involving the intestine is not safe, i.e. the surgeon cannot see well or the surgeon cannot determine if an enterotomy has occurred, the patient should be opened! Deaths have been reported from laparoscopic incisional hernioplasty due to bowel injuries that have not been perceived during surgery and only become apparent postoperatively. By the time the diagnosis is made, the patients are septic and succumb to this complication.
Discussion
Initially described in 1992, laparoscopic repair of incisional hernias has evolved from an investigational procedure to one that can safely and successfully be used to repair ventral hernias. The well-established benefits of laparoscopy repair are less postoperative pain, reduced hospital stay and recovery time, low complication and recurrence rates based on numerous reports, meta-analysis, and few randomized trials. Conventionally, the laparoscopic ventral hernia repair (LVHR) entails the intraperitoneal placement and fixation of the prosthetic mesh. An alternative technique has been tried in a few studies and proposed and to be an advancement of the conventional approach.
Despite its significant prevalence and associated morbidity, there is little in the way of evidence-based guidelines regarding the timing and method of repair of ventral and particularly, incisional hernias. Several large studies on laparoscopic ventral hernia repair (LVHR) have been reported. This technique has proven to be a safe and feasible alternative to open mesh repair. Although many are retrospective series and a few comparative studies, only two completed randomized trials comparing open versus laparoscopic mesh repair have been published. Based on these studies, LVHR has been found to have shorter operating time depending on the surgeon’s experience, shorter hospital stay, and lower complication rates especially wound and mesh infections, and lower recurrence rate during the follow-up period. This evidence has led to the suggestion that now; it would be unethical to conduct a prospective randomized controlled trial comparing LVHR and open approach.
Laparoscopic ventral hernia repair (LVHR) techniques are based on the fundamental principles of the open preperitoneal repair described by Stoppa and Rives. The placement of a large mesh in the preperitoneal location allows for an even distribution of forces along the surface area of the mesh, which may account for the strength of the repair and the decreased recurrence associated with it. The repair capitalizes on the physics of Pascal’s principle of hydrostatics by using the forces that create the hernia defect to hold the mesh in place. For this to attain maximum effect, there has to be a wide mesh overlap over the defect and adequate, secure fixation. In the open approach, attaining an overlap of 3 to 5 cm required extensive soft tissue dissection, with the resultant increase in wound complications. Larger defects should require more overlap and smaller ones theoretically less. The laparoscopic approach not only allows a clear definition of the defect margins but also the identification of additional defects that may not have been clinically apparent preoperatively.
Both the inlay and onlay placements of prosthetic mesh embrace these fundamental principles of hernia repair. The onlay and the transabdominal inlay methods, allow for an adequate diagnostic laparoscopy to clearly define the margins and the number of the hernia defects including the occult ones.
Mesh Placement and Fixation
One of the critical technical points that significantly impact on any method of hernia mesh repair is adequate mesh fixation. The mesh is held in position by sutures and /or staples, clips, tacks, intra-abdominal pressure, and later by fibrinous growth. The most widespread technique in onlay approach involves the fixation of mesh with tacks and transabdominal permanent sutures. Some surgeons have tried to reduce the operating and possibly postoperative discomfort by reducing or eliminating the use of sutures. The physics of mesh fixation does not support the sole placement of tacks. The majority of the meshes used are about 1 mm thick. A perfectly placed tack can be expected to penetrate only 2 mm beyond the mesh thus tacks will not give the same holding strength as full-thickness abdominal wall suture. Furthermore, the mesh is placed against the peritoneum, so any ingrowth is most likely into the peritoneum and not into the fascia.
A detachment of tacks has also been attributed to some recurrence of a hernia. Postoperative recurrence of ventral hernia repair is reported to be as high as 13 percent when only a stapling, clipping or the tacking device is used for mesh fixation. Proper use of the transfascial fixation sutures in combination with staples decreased the recurrence rate to as low as 2 percent. Therefore the current recommendation for mesh fixation is that a transfascial suture should be placed at a distance of 5 cm each along the perimeter of the mesh and tacking devices be used to affix the edge of the mesh at 1 cm intervals. The preperitoneal approach mesh fixation differs in that, there is immediate and continued fixation by the intact peritoneal sac and whether tacks or sutures or both are used, they fix the mesh directly onto the fascia. The primary concern of the peritoneal flap in the inlay technique is to achieve secure fixation of the mesh to the underlying fascia. The fibrinous ingrowth is from the fascia and not the peritoneum. Furthermore, the preperitoneal positioning confers with the original design of Stoppa.
Perhaps the most compelling advantage of the preperitoneal placement of the mesh in the inlay approach is the avoidance of direct interaction between the mesh and the intra-abdominal viscera. Contact of the viscera with foreign material such as the prosthesis may lead to an inflammatory response and adhesion formation which can induce chronic pain, intestinal obstruction, enterocutaneous fistula, and infertility. In addition, adhesions complicate any future intra-abdominal surgery. The peritoneal covering also allows the use of conventional meshes, which have been associated with intense inflammatory response and adhesion formation by some workers. The choice of the mesh used in LVHR may be the most contentious issue, particularly when the financial cost is a major consideration.
The biomaterials available for ventral hernia repair have undergone many changes over the last several years. There are new products that have either been recently introduced or are in developmental stages. All seek to achieve two goals; rapid and permanent ingrowth into the body wall and diminution of the risk of intestinal adhesions while maintaining its tensile strength. The visceral side should be smooth, nonerosive antiadhesive, and not easily susceptible to infection. This visceral barrier should be present for at least one week because this is the time frame in which adhesions form. The ventral side should be macroporous allowing for fibroblast in growth and a foreign body reaction may be necessary for incorporation and high tensile strength.
Polypropylene (prolene) mesh, introduced by Sir Francis Usher in 1958 and modified in 1962 has gained widespread popularity and several types are commercially available today. The polyester mesh was introduced in Europe in the 1950s. Stoppa used the polyester mesh in their landmark article describing preperitoneal repair of ventral hernia in 1989. Prolene mesh is currently the most widely used because it is relatively inexpensive, easy to handle, has a memory (allows them to regain the original shape), and is firmly incorporated in the abdominal wall due to its ability to induce an intense inflammatory reaction. A 2 to 5 percent fistula rate has been reported with polypropylene mesh used intra-abdominally leading to the suggestion the great care must be taken to separate it from the bowel if it has to be used at all. However, some studies do not support this view. Bingener found no association of visceral adhesion when prolene was used with adequate omental interposition between it and the bowel. In another study involving 136 patients, Vrijland concluded that enterocutaneous fistula appears to be very rare after prolene mesh repair regardless of intraperitoneal placement, omental coverage or closing
the peritoneum.
A study comparing the biomaterials used in LVHR found polyester to have the highest incidence of infection, fistulization, and recurrence. The expanded polytetrafluoroethylene (ePTFE) has the longest history in the use for these hernias repair. The original description of the procedure used an early generation of the ePTFE product. The current product has one smooth surface with 3 microns ePTFE interstices, while the other side has 22 microns interstices to facilitate fibroblastic ingrowth for firm fixation. Other modifications of this product involve the incorporation of antimicrobials on the visceral surface. All of the composite prostheses have ePTFE and prolene or polyester but differ in the number and attachment of them together. There are no reports of intestinal fistulization or obstruction with ePTFE though it has also been found to induce inflammation and fibrosis in laboratory animals.
However, the use of synthetic materials is not without problems. As a foreign material, the repair site is subjected to inflammation, susceptibility to infection, and pain as a foreign body response. The encapsulation could affect the elastic function of the abdominal wall and the aesthetic outcome of the repair. This has stimulated the search for natural biological prostheses like surgisis, collagen, glycosaminoglycans from porcine intestinal submucosa, and alloderm. The financial cost to the clinical-benefit ratio for use of the substantially expensive composite meshes is unquantified and is likely to remain as such because, given the widespread acceptance of composite products, randomized, clinical comparison with prolene is unlikely to occur. Therefore, in selected circumstances, it may be acceptable to use a simple mesh, if this can be excluded from the bowel by tissue interposition be it omentum or peritoneum. A composite mesh should be considered as the current standard of care.
The extraperitoneal placement of the prostheses would in principle diminish the intra-abdominal complications associated with the formation of adhesions. It would also allow the safe use of conventional meshes like prolene, which has high intrinsic tensile strength, good memory, and cheaper. In addition, the peritoneal coverage over the entire mesh provides additional security of fixation and a better mechanical advantage. As such it can be seen as an advance over the onlay approach. However, the placement is technically demanding as evidenced by the high iatrogenic peritoneal tears in the largest series and it may not be feasible in the scarred abdomen of incisional and recurrent hernias, which constitute the bulk and seems to benefit most, from LVHR. Thus the issue of limitation of patient population amongst the technical feasibility and adequacy of defect coverage are issues of great concern before the method is accepted as an additional procedure for LVHR.
The good results and the attributed safety of LVHR are based on the large number of studies mainly utilizing the intraperitoneal approach. The generalization of the procedure has resulted in multiple variations of techniques. Overall, fewer complications are reported after LVHR than after open mesh repair especially in relation to wound and mesh infection. The efficacy of the inlay approach as an advancement of the conventional repair needs to be evaluated in terms of the several specific complications that are of particular relevance in laparoscopic procedures.
Bowel Injury
Probably the most dreaded complication is bowel injury and particularly if it is missed intraoperatively. It is a potentially lethal complication. The overall incidence of bowel injury does not differ significantly between open repair and laparoscopic repair and is generally low with either approach (1–5% when serosal injuries are included). Pneumoperitoneum may hinder the recognition of bowel injury at the time of operation. There have also been reports of late bowel perforation secondary to thermal injury with laparoscopic repair.
Minimizing the use of electrocauterization and ultrasonic dissection markedly reduces the risk of bowel injury. The visualization afforded by the pneumoperitoneum place adhesions between the abdominal wall and the bowel under tension. The high-intensity light source and the magnification inherent in the laparoscopy facilitate the identification of the least vascularized planes. As far as possible, direct grasping the bowel should be avoided preferring simply to push it or to grasp the adhesions themselves to provide counter traction. External pressure on the hernia may also help. Larger vessels in the omentum or adhesions are controlled with clips. Some degree of oozing from the dissected areas is tolerated; such oozing almost always settles down without specific hemostatic measures.
Adhesion
In cases of dense adhesions, it is preferable to divide the sac or the fascia rather than risk injury to the bowel. Densely adherent polypropylene mesh is best excised the abdominal wall rather than attempting to separate it from the serosa of the bowel. If bowel injury is suspected immediate and thorough inspection should be carried out. It may be difficult or impossible to find the exact site of injury later once the bowel has been released and freed of its attachments. Once the injury is recognized, it is the surgeon’s level of comfort with laparoscopic suture repair determines the best approach. With minimal spillage of bowel contents, the injury may be treated with either laparoscopic repair or open repair; the latter usually can be carried out through a mini-laparotomy over the injured area. Whether the mesh prosthesis is put primarily or later depends on the degree of contamination. More significant bowel injuries necessitate conversion to open repair. Missed injuries manifest postoperatively mandating re-exploration with occasional removal of the mesh and immediate recurrence of the hernia.
Infection
One of the greatest benefits of LVHR is the reduction in wound and mesh infections. In a detailed analysis of wound complications from a pooled data of forty-five published series involving 5340 patients, Pierce reported wound infection rates of 4.6 to 8 times to fold higher in open versus LVHR. The number of mesh infections was also significantly higher with open approaches. Wound problems are strongly linked with soft tissue dissection required for retro muscular placement of large pieces of mesh. The intraperitoneal approach obviates the need for this dissection that potentially devascularizes the fascia and causes hematoma formation, both of which contribute to infection. Although the incidence of mesh infection is very low the consequences are severe. Infections of prolene meshes can be managed locally with surgical drainage and excision of exposed, unincoporated segments but that of ePTFE requires removal in most cases due to its relatively low incorporation onto the body wall. Removal of the mesh results in a return of the defect and its added morbidity. An analysis of all series with more than 50 patients indicated a mesh infection rate of 0.6 percent, cellulitis of the trochar sites that resolved on antibiotics alone in 1.1 percent, and an overall wound and mesh complications of 1.7 percent. This has led to the widely perceived conclusion that the most compelling argument for LVHR is the minimization of soft tissue dissection and the associated reduction in the morbidity of local wound complications and potential infection of the implanted mesh. The high mesh infection rate reported in the inlay approach could be related to the extensive dissection of the peritoneal flap.
Seroma
Seroma formation is one of the most commonly reported complications in LVHR though it is not unique to laparoscopy. It swelling like mass occurs immediately after the operation in virtually all patients but disappears after a few weeks. Most seromas develop above the mesh and within the retained hernia sac. The mean incidence of seroma in reported series at a range of 4 to 8 weeks is 11.4 percent. In the largest multi-institutional trial, seromas that were clinically apparent more than 8 weeks were considered a complication and occurred in 2.6 percent. Regardless of whether they are aspirated under sterile conditions or allowed to resolve, they rarely cause long-term morbidity. Aspiration may increase the risk of mesh infection but is recommended if they enlarge or persist before they reach their extremes. Patients sometimes mistake a tense seroma for recurring incisional hernia, but appropriate preoperative discussion should provide them with significant reassurance on this point.
Although Feldman suggests that, seroma formation is not related to a particular type of mesh, Carbanjo and Heniford reported a higher incidence of seroma formation with ePTFE than prolene based meshes. The low incidence in the latter meshes has been attributed to the large pores of the prolene-based meshes that allow more efficient resorption of wound secretions into the abdominal cavity than ePTFE meshes. The dissection of the preperitoneal space during the inlay method may lead to more seroma formation. This is supported by the fact that, in the classical description of the onlay technique, it is emphasized that no attempts should be made to reduce or resect the hernia sac. This has been established to be unnecessary and to increase the incidence of seroma formation. The peritoneum interposed a barrier between the mesh and the abdominal cavity may hinder the direct drainage of this fluid regardless of the mesh used. Thus based on these facts, it seems plausible that the problem of seroma formation is expected to be higher in the inlay than the conventional onlay approach.
Postoperative Pain
After LVHR, about 5 percent of patients complain of persistent pain and point tenderness at the transabdominal suture site which usually resolves spontaneously within 6 to 8 weeks. If it does not, the injection of local anesthetic into the area around the painful suture has good results. Since missed enterotomy is a grave concern in LVHR, particularly after a difficult adhesiolysis, a correct interpretation of the significance of postoperative pain is an important issue. Whether or not to re-laparoscope a patient who experiences severe pain remains an important issue. A possible explanation of the common type of pain may be that the transabdominal suture entraps an intercostal nerve as it courses through the abdominal muscles. Local muscle ischemia may be another possibility. As such, it is an unavoidable adverse outcome of either approach so long as there is suture fixation of the prosthetic mesh. Whether it can be avoided by not using suture in the preperitoneal approach has to be weighed against the clinical benefit ratio of such a repair.
Obesity
The morbidly obese population represents a significant population of patients who present for ventral hernia repair. The advantages of minimal dissection, smaller wounds, and decreased wound complications using the onlay methods have been concluded in a recent review and all mitigate against the preperitoneal dissection of the inlay approach.
Recurrence
The ultimate measure of the effectiveness of hernia surgery is the recurrent rate. Recurrence rates after LVHR range from 1.1 to 13 percent whereas those after the open repairs ranged from 25 to 49 percent. In a multicenter series of 850 cases, the recurrence rate after a mean follow-up period of 20 months was 4.7 percent. The average recurrent rates using the onlay approach are approximately 4.2 percent although rates as high as 17 percent have been reported. The critical technical points related to recurrence are inadequate mesh fixation particularly with sutures and prostheses that overlap the defect by less than 2 to 3 cm. Other factors associated with high recurrent rates include postoperative complications, previous repairs, missed hernias as in the “Swiss cheese” defects, longer operating time, and obesity. The surgeon’s level of experience plays a significant role in patient outcome, as demonstrated by a group that compared the outcomes for their first 100 laparoscopic incisional hernia repair patients, with those for their second 100. Recurrence rates after a mean follow-up period of 36 months dropped from 9 percent in the first 100 patients to 4 percent in the second 100. In addition, the second set of patients were an average of 9 years older, had a higher percentage of recurrent hernias, and exhibited more comorbidities, yet despite these added challenges, operating time was not lengthened, length of stay was similarly short, and the complication rate was no different. A multivariate analysis of these variables indicated that prior failed hernia and increased estimated blood loss predicted recurrence while the other variables included; body mass index, defect size, and the size of the mesh did not have a positive correlation.
Although the results of large randomized trials are not available yet, the recent evidence to date suggests that the conventional onlay laparoscopic approach to the repair of ventral hernias is highly promising. The proposed laparoscopic preperitoneal placement of prostheses seems to negate most of the positive attributes of LVHR in most ways. This technique may be advantageous in small primary hernias, in a highly selected patients population. However, the widespread application of this approach or even the possibility of it being entered into a randomized trial appears dismal in the prevailing evidence and the patient's population that usually present with this structural disability.
Introduction
Ventral hernias refer to fascial defects of the anterolateral abdominal wall through which intermittent or continuous protrusion of abdominal tissue or organs may occur. They are either congenital or acquired. In adults, more than 80 percent of ventral hernias result from previous surgery hence the term incisional hernias. They have been reported to occur after 0 to 26 percent of abdominal procedures. Although these hernias mostly become clinically manifest between 2 to 5 years after surgery, studies have shown that the process starts within the first postoperative month. They are said to occur as a result of a biomechanical failure of the acute fascial wound coupled with clinically relevant impediments to acute tissue repair and normal support function of the abdominal wall.
Historically, incisional hernias have been repaired with either primary suture techniques or placement of a variety of prosthetic materials. Before the 1960s, most ventral hernias were repaired primarily with suture and a few with metallic meshes. Even with some modifications, recurrence rates with the primary suture repair ranged from 24 to 54 percent. The introduction of polypropylene mesh repair by Usher in 1958 opened a new era of tension-free herniorrhaphy. Recurrence rates with prosthetic mesh decreased to 10 to 20 percent. Subsequently, it was realized that the placement and fixation of the mesh were more crucial in determining the outcome of the repair. The placement of the mesh in the preperitoneal, retro muscular position with a wide overlap of at least 5 cm over the hernia defect in all directions was introduced in the late 1980s. The refinement of this method decreased the recurrence rates to as low as 3.5 percent making it to be declared the standard of care of ventral hernias. However, implantation of the mesh by open techniques requires a wide dissection of soft tissue contributing to an increase in wound infection and wound-related complications.
Ventral hernia results from a weakness in the musculoaponeurotic layer of the anterior abdominal wall. This type of hernia has the root of development during the period of development like; omphalocele, gastroschisis, and congenital umbilical hernia. Recently, the ventral hernias are reported more due to the iatrogenic factor. Even after laparoscopic surgery, if the 10 mm port is not repaired properly there is always a chance of ventral hernia (Incisional hernia) development. Obviously the initial closure is the most important since faulty technique will universally lead to the development of herniation. There are other associated co-morbid conditions, which may encourage the creation of incisional herniation. These include intra-abdominal or wound infection, morbid obesity, steroid use, previous use of the incision, hematoma formation, and respiratory compromise with an increased cough. Other factors include the duration of the operation, crossing incisions, ineffective wound drainage, and excessive wound tension. Two other important variables include nutritional aspects as well as the presence of cancer which overall reduces the ability for wound healing and collagen deposition in the wound.
The repair of incisional and ventral hernias continues to be a surgical challenge. Reports published in the medical literature indicate 3 to 13 percent of laparotomy patients develop incisional hernias. Moreover, clinical studies indicate that the traditional, or open, a technique to repair large abdominal wall defects is associated with recurrence rates ranging from 25 to 49 percent.
Among the non-iatrogenic ventral hernias, divarication of rectus abdominis, umbilical, para-umbilical, Spigelian, and epigastric are more common. In 1992, a successful series of laparoscopic incisional hernia repairs were reported in the medical literature. Since then, the technique has been refined and has grown in acceptance within the surgical community.
The laparoscopic technique for ventral hernia repair involves the placement of a tension-free prosthetic bridge across the musculofacial defect rather than attempting to approximate the edge of the defect. The hernia defect is covered by an appropriate size of mesh once the content of the sac is reduced. Most of the time sac content is omentum. Sometime omentum has adhered so tightly that electrosurgical dissection with the help of bipolar is essential. Recently, a much newer type of mesh is available in which PTFE and polypropylene are more popular. There was always a fear of bowel adhesion and fistulization with the use of polypropylene mesh but the clinical evidence of thousands of surgery has suggested that the omental adhesion is expected but bowel adhesion is not common and intraperitoneal placement of polypropylene mesh is quite safe. Almost all types of ventral hernia can be repaired by minimal access surgical approach. Hernias like multiple defects (Swiss cheese hernias) are greatly benefited by this approach as all defects get directly visualized and appropriately covered by single mesh. Contraindication of laparoscopic repair of ventral hernia is a very large hernia with a huge protrusion of skin which is thin enough. Skinfold is necessary to correct by abdominoplasty. Dense intra-abdominal adhesions are also a relative contraindication of laparoscopic repair of ventral hernia.
Laparoscopic Anatomy
A ventral hernia develops due to the structural weakness of the abdominal wall. The muscle and fascia that span the space between the costal margin superiorly, the spine and muscles of the back posteriorly, and the pelvis inferiorly support the abdominal wall by giving strength. The parietal peritoneum in ventral hernia extends into the defect to form the sac. Adhesions to adjacent viscera must be divided to define the defect.
Operative Procedure
The patient should be clearly informed that laparoscopic repairs will not help cosmetically if the skin is lax and hanging loosely. Bowel preparation is a good practice to have more room inside the abdominal cavity to handle the instrument. After anesthesia, a nasogastric tube is a must to deflate the stomach completely because in most cases access should be through left hypochondria. Splenohepatomegaly is an absolute contraindication of the access through left hypochondrium. The patient is placed in a supine position without any tilt of the operation table so that bowel is distributed evenly.
Position of Surgical Team
The surgeon stands left to the patient with a camera operator on his left or right side depending upon the location of the ventral hernia. If a ventral hernia is below the umbilicus, the camera operator stands right to the patient. If the defect is above the umbilicus, the camera operator should stand left to the patient. The monitor should be placed opposite to the surgeon and the instrument trolley should be towards the leg of the patient.

Operating room set up in ventral hernia repair
Port Position
The technique of laparoscopic repair of ventral hernia is quite simple. The first pneumoperitoneum is created at a site away from the defect. Three port techniques are used for laparoscopic repair of ventral hernia. The first step in performing laparoscopic repair of ventral hernia is gaining access to the free peritoneal cavity. A site distant from any prior incision and the hernia defect is chosen. Typically this is in the right upper quadrant (RUQ) or left upper quadrant (LUQ). The absence of incisions in these locations does not necessarily guarantee the absence of adhesions to viscera. While many approaches for access to the peritoneal cavity have been described, including blind insufflation and specialty trocars, open access in the fashion of Hasson is by far the safest alternative.
Once the pneumoperitoneum is created all other port is placed according to the baseball diamond concept. The most preferred site of access is the left hypochondrium in the most midline and lower abdominal defects.
First access should be preferably through left hypochondria if the Veress needle technique is used and then two other ports should be made so that a proper triangle is formed. The distance between the two ports should not be less than 5 cm. The telescope will first enter through the left hypochondriac port but once dissection starts the telescope will come in the middle so that the angle between two working port will become 60°. The 10 mm 30° telescope is better to view the anterior abdominal wall. The ventral hernia repair can be performed by two techniques. The first technique is the intraperitoneal or Onlay mesh technique in which mesh is placed without dissecting peritoneum. This is also called the Onlay method. All content of the sac is reduced and any adhesion (if present) is cleared. An appropriate size of the mesh is then inserted.
After free access to the peritoneal cavity is obtained. This also represents the greatest risk to the patient. The difficulty of adhesiolysis is unpredictable, although the presence of polypropylene mesh should be a red flag indicating the potential for the presence of dense and difficult to dissect adhesions, often involving the bowel. All maneuvers performed as part of the lysis of adhesions must be done under direct vision. This is best carried out by sharp dissection utilizing bimanual palpation of the anterior abdominal wall, placing the adhesions under variable degrees of tension. There is significant risk in extensive blunt dissection, as the bowel may be fixed at several points placing it at risk for unrecognized perforation with the tip of dissecting instruments. In spite of the enthusiasm for different energy sources, these are best avoided. As in open cases, dissection should be carried out at the avascular junction of the adhesions and the anterior abdominal wall. Ligating clips or the limited application of an energy source can be used when significant bleeding or vessels are encountered. In the majority of cases, even this is unnecessary. The risk of monopolar cautery is well known, but there is also a risk of thermal injury by direct contact with ultrasonic or radiofrequency dissection instruments. This is particularly true in the poorly visualized area behind adhesions.

Bipolar can be used safely in case of adhesion with omentum
If omentum adheres to the anterior abdominal wall it should be dissected after applying bipolar or extracorporeal knot. It is critical that all adhesions to the anterior abdominal wall be released to allow adequate patch placement and fixation. Once adhesiolysis has been completed, the exact extent of the defect can be directly evaluated. The defects are carefully drawn onto the skin of the anterior abdominal. In the case of multiple defects, the area drawn should include all of the defects. We have progressed to repairing the entire area of a previous incision as opposed to simply repairing a single defect. There have been a number of patients who have presented later in follow-up and are discovered to have a new hernia, outside the area of the previous repair. In open surgery, these may have simply been considered recurrences. If there is any difficulty in delineating the margins of the defect, a spinal needle can be passed perpendicular to the anterior abdominal wall and through the margins of the defect.
The selection of the size of the mesh is important to prevent recurrence of hernia and it should be sufficiently big so that approximately 4 cm healthy margin of defect of hernia should be covered all around. Recently new hybrid mesh has been introduced with absorbable material on one side and unabsorbable proline on the other. These meshes are better than proline because adhesion is less likely to develop due to absorbable material towards the bowel.
The mesh is fixed along margins and around the ring of defect of rectus to ensure a close approximation of mesh to the abdominal wall. Care should be taken that mesh should not be corrugated and it should be in proper contact with the anterior abdominal wall. Tacker, Protack, or Endo anchor can be used to fix the mesh. After fixing the mesh greater omentum is speeded like an apron in between the bowel and mesh. Some adhesion of mesh with omentum is always expected in this technique. If a patient is early mobilized and a newer generation of mesh is used the long-term complication of adhesion is very less with this technique.

Fixation of the mesh can be accomplished with endoanchor
Loosely held mesh hanging through the anterior abdominal wall will definitely increase the chance of adhesion with bowel. Tacker should be used to fix the mesh in position. Recently, a technique of using proline suture to fix the mesh with the anterior abdominal wall is used with the help of a suture passer or looping technique with the help of a Veress needles cannula. The main idea of this method is to reduce the cost of surgery, but there is an increased chance of infection and adhesion with this method. We also lack any long-term randomized controlled trial to prove the outcome of this external suture technique to fix the mesh in ventral hernia repair.
Choice of Mesh in Ventral Hernia
Synthetic Materials
A variety of synthetic polymeric meshes were developed in the second half of the 20th century and revolutionized hernia repair. With these meshes, abdominal wall defects could be repaired without undue tension on the sutured tissue, decreasing the high recurrence rates of abdominal wall hernia repair. Sir Francis Usher introduced woven monofilament polypropylene mesh in 1958. It was modified to a knitted mesh in 1962 so that the mesh would not unravel when it was cut. Polypropylene mesh gained widespread popularity over the next 30 years and several types of polypropylene mesh are commercially available today. The polyester mesh was also introduced in the 1950s in Europe. Rives and Stoppa employed polyester mesh in their landmark article describing a preperitoneal technique of ventral hernia repair in 1989. The technique described by Rives and Stoppa has become the standard by which all abdominal wall incisional hernia repairs are measured. Polypropylene mesh and polyester mesh revolutionized abdominal wall repair because the meshes did not deteriorate with age, were pliable, and would stretch, allowing for more even load distribution. Nevertheless, the large interstices in polypropylene and polyester mesh promoted adhesion formation when the mesh came into contact with the visceral abdominal cavity. Reported complications included small bowel obstruction, erosion, and fistulization. Expanded polytetrafluoroethylene (ePTFE), initially used as a vascular prosthesis, was adapted for abdominal wall incisional hernia repair in 1983 by WL Gore and Associates and modified several times in the 1990s. Unlike the polypropylene and polyester meshes that preceded it, ePTFE is microporous and select products are uniquely designed with pores measuring 3 microns on the visceral side facing the abdominal cavity and 22 microns facing the abdominal wall. This design promotes fibroblastic and vascular ingrowth from the abdominal wall 22-micron side but inhibits tissue attachment to the material on the 3-micron side when exposed to the intraabdominal cavity. There are no reports of fistulization or small bowel obstructions due to adhesions from ePTFE material.
Synthetic meshes, made of materials such as ePTFE (expanded polytetrafluoroethylene) and polypropylene is used most of the time for repair of the hernia. The repair process for these materials is based on scar formation in and around the mesh. The advantage of using these materials is that they generally do not react with human tissue. They are strong and do not tear easily, are readily available, inexpensive, and have a long history of being used for soft tissue replacements.
However, the use of synthetic materials is not without problems. As a foreign material, the body may react to its presence by growing around it (encapsulation) in an attempt to exclude it from the body. In the process, tissue forms a capsule of rigid, fibrous scar tissue around the synthetic material. The rigid capsule could affect the function and the aesthetic outcome of the repair. Furthermore, foreign bodies such as synthetic materials increase the risk of infection when implanted in the body. As part of the foreign body response, the repair site may be subjected to inflammation, infection, and pain.
Surgisis
It is porcine intestinal submucosa and specifically designed as a surgical graft for hernia and abdominal body wall repair. Surgisis gold combines strength with flexibility in a naturally occurring graft material that allows for hernia repair without the need for a permanent synthetic prosthesis. Surgisis® Gold™ supports the surgical site while the body's natural healing process replaces the graft with new host tissue. It is collagen biomatrix, naturally occurring and acellular with 18 months shelf life.
Alloderm
It is a biological dermal matrix from processed donated human tissue. AlloDerm is processed from donated human skin. The tissue goes through a cell removal process while retaining the important biochemical and structural components. AlloDerm is, thus, acellular human tissue. Since AlloDerm is derived from human tissue, there may be a concern that it might harbor disease-carrying viruses. Tissue donors are screened and tested for transmissible diseases including HIV, hepatitis, and syphilis before tissue processing. AlloDerm has been utilized in more than 7,50,000 implants and grafts to date, without any reported incidence of viral disease transmission to a patient. AlloDerm repairs damaged tissue by providing a foundation for new tissue regeneration. The skin components preserved in AlloDerm contain the information that will help your own tissue to grow into the AlloDerm after placement at the repair site.
Proceed
Soft PPM covered with PDS and oxidized regenerated cellulose fabric.
The Second Technique
Preperitoneal Repair of Ventral Hernia
This technique is also called inlay technique of laparoscopic ventral hernia repair. The peritoneum is incised and preperitoneal space is created in this technique. The sac of the hernia if excised nicely. It can give some extra flap of peritoneum for the successful overlapping of both the edges. In this technique margin of defect is sutured intracorporeally to decrease the gap. Pressing the abdominal wall on both the side from outside will help to obliterate the space in this hernia surgery. Either intracorporeal stitches or external mattress sutures can be used to fix the mesh to the musculofacial defect. Once the mesh is fixed, the peritoneum is sutured using vicryl to cover the mesh. This method of laparoscopic ventral hernia repair is the same as that of open surgery and it is supposed that the formation of adhesion is less.
Complications
• Bowel adhesion
• Fistulization
• Nerve injury
• Vascular injury.
Multiple studies have documented that open hernioplasty has significant morbidity. Leber reported a 27 percent long- term complication rate with the open repair; among them being infection, hematoma, and seroma, chronic sinus tract formation, mesh extrusion, fistula formation as well as soft tissue problems such as a non-healing wound. White reported 34 percent of 250 open ventral hernia repairs had wound-related complications. The complications of open repair mainly relate to the type of mesh that is most commonly used (polypropylene and polyester meshes). In addition, the wide dissection of soft tissue that is required for a Stoppa type retro-rectus repair or a Chevrel type anterior repair leads to the many wound-related problems. Some patients will develop a fluid collection, which is commonly called a seroma, between the mesh and the abdominal wall. Many of these are not apparent to the patient or the surgeon but some are evident and can be bothersome to the patient. Complications from these seromas were sometimes reported in many studies. Most surgeons do not aspirate these fluid collections for fear of infecting the prosthetic. However, the author has freely aspirated the seromas if they are large or if they are bothersome to the patient. The author has never seen an infection of the prosthetic from aspiration of these fluid collections if full aseptic precaution is taken.
Probably the most dreaded complication that has been seen is bowel injury. Enterotomy is a well-documented complication and commonly occurs and can be readily visualized and handled through an incision. Laparoscopy presents a whole new situation with respect to enterotomy. Prevention is the first line of defense. The lysis of adhesions is well visualized due to the magnification and high-intensity light source inherent in the laparoscopic technique. It is very important that energy sources be used very sparingly if at all during lysis of adhesions. If a surgeon enters the proper planes, there is very little bleeding and thus a low need for energy sources. Inappropriate use of energy sources is a common cause of unrecognized enterotomy. Monopolar cautery has the problem of current spread, and it is easy to coagulate one area and see the current spread to the adjacent area instantaneously. For this reason, monopolar cautery should not be used adjacent to the bowel. The ultrasonic or radiofrequency dissection instruments are "sold" with the supposed advantage that there is minimal thermal spread, unlike monopolar cautery. Although this may be true, the tip remains very hot and any touching of viscera can cause a burn that may not be apparent during the operation. It is only after several hours, either that night or the next day, when the tissue sloughs, that the enterotomy presents itself. We do not recommend the use of ultrasonic or radiofrequency dissection instruments for this reason. The most important thing to remember is that if lysis of adhesions involving the intestine is not safe, i.e. the surgeon cannot see well or the surgeon cannot determine if an enterotomy has occurred, the patient should be opened! Deaths have been reported from laparoscopic incisional hernioplasty due to bowel injuries that have not been perceived during surgery and only become apparent postoperatively. By the time the diagnosis is made, the patients are septic and succumb to this complication.
Discussion
Initially described in 1992, laparoscopic repair of incisional hernias has evolved from an investigational procedure to one that can safely and successfully be used to repair ventral hernias. The well-established benefits of laparoscopy repair are less postoperative pain, reduced hospital stay and recovery time, low complication and recurrence rates based on numerous reports, meta-analysis, and few randomized trials. Conventionally, the laparoscopic ventral hernia repair (LVHR) entails the intraperitoneal placement and fixation of the prosthetic mesh. An alternative technique has been tried in a few studies and proposed and to be an advancement of the conventional approach.
Despite its significant prevalence and associated morbidity, there is little in the way of evidence-based guidelines regarding the timing and method of repair of ventral and particularly, incisional hernias. Several large studies on laparoscopic ventral hernia repair (LVHR) have been reported. This technique has proven to be a safe and feasible alternative to open mesh repair. Although many are retrospective series and a few comparative studies, only two completed randomized trials comparing open versus laparoscopic mesh repair have been published. Based on these studies, LVHR has been found to have shorter operating time depending on the surgeon’s experience, shorter hospital stay, and lower complication rates especially wound and mesh infections, and lower recurrence rate during the follow-up period. This evidence has led to the suggestion that now; it would be unethical to conduct a prospective randomized controlled trial comparing LVHR and open approach.
Laparoscopic ventral hernia repair (LVHR) techniques are based on the fundamental principles of the open preperitoneal repair described by Stoppa and Rives. The placement of a large mesh in the preperitoneal location allows for an even distribution of forces along the surface area of the mesh, which may account for the strength of the repair and the decreased recurrence associated with it. The repair capitalizes on the physics of Pascal’s principle of hydrostatics by using the forces that create the hernia defect to hold the mesh in place. For this to attain maximum effect, there has to be a wide mesh overlap over the defect and adequate, secure fixation. In the open approach, attaining an overlap of 3 to 5 cm required extensive soft tissue dissection, with the resultant increase in wound complications. Larger defects should require more overlap and smaller ones theoretically less. The laparoscopic approach not only allows a clear definition of the defect margins but also the identification of additional defects that may not have been clinically apparent preoperatively.
Both the inlay and onlay placements of prosthetic mesh embrace these fundamental principles of hernia repair. The onlay and the transabdominal inlay methods, allow for an adequate diagnostic laparoscopy to clearly define the margins and the number of the hernia defects including the occult ones.
Mesh Placement and Fixation
One of the critical technical points that significantly impact on any method of hernia mesh repair is adequate mesh fixation. The mesh is held in position by sutures and /or staples, clips, tacks, intra-abdominal pressure, and later by fibrinous growth. The most widespread technique in onlay approach involves the fixation of mesh with tacks and transabdominal permanent sutures. Some surgeons have tried to reduce the operating and possibly postoperative discomfort by reducing or eliminating the use of sutures. The physics of mesh fixation does not support the sole placement of tacks. The majority of the meshes used are about 1 mm thick. A perfectly placed tack can be expected to penetrate only 2 mm beyond the mesh thus tacks will not give the same holding strength as full-thickness abdominal wall suture. Furthermore, the mesh is placed against the peritoneum, so any ingrowth is most likely into the peritoneum and not into the fascia.
A detachment of tacks has also been attributed to some recurrence of a hernia. Postoperative recurrence of ventral hernia repair is reported to be as high as 13 percent when only a stapling, clipping or the tacking device is used for mesh fixation. Proper use of the transfascial fixation sutures in combination with staples decreased the recurrence rate to as low as 2 percent. Therefore the current recommendation for mesh fixation is that a transfascial suture should be placed at a distance of 5 cm each along the perimeter of the mesh and tacking devices be used to affix the edge of the mesh at 1 cm intervals. The preperitoneal approach mesh fixation differs in that, there is immediate and continued fixation by the intact peritoneal sac and whether tacks or sutures or both are used, they fix the mesh directly onto the fascia. The primary concern of the peritoneal flap in the inlay technique is to achieve secure fixation of the mesh to the underlying fascia. The fibrinous ingrowth is from the fascia and not the peritoneum. Furthermore, the preperitoneal positioning confers with the original design of Stoppa.
Perhaps the most compelling advantage of the preperitoneal placement of the mesh in the inlay approach is the avoidance of direct interaction between the mesh and the intra-abdominal viscera. Contact of the viscera with foreign material such as the prosthesis may lead to an inflammatory response and adhesion formation which can induce chronic pain, intestinal obstruction, enterocutaneous fistula, and infertility. In addition, adhesions complicate any future intra-abdominal surgery. The peritoneal covering also allows the use of conventional meshes, which have been associated with intense inflammatory response and adhesion formation by some workers. The choice of the mesh used in LVHR may be the most contentious issue, particularly when the financial cost is a major consideration.
The biomaterials available for ventral hernia repair have undergone many changes over the last several years. There are new products that have either been recently introduced or are in developmental stages. All seek to achieve two goals; rapid and permanent ingrowth into the body wall and diminution of the risk of intestinal adhesions while maintaining its tensile strength. The visceral side should be smooth, nonerosive antiadhesive, and not easily susceptible to infection. This visceral barrier should be present for at least one week because this is the time frame in which adhesions form. The ventral side should be macroporous allowing for fibroblast in growth and a foreign body reaction may be necessary for incorporation and high tensile strength.
Polypropylene (prolene) mesh, introduced by Sir Francis Usher in 1958 and modified in 1962 has gained widespread popularity and several types are commercially available today. The polyester mesh was introduced in Europe in the 1950s. Stoppa used the polyester mesh in their landmark article describing preperitoneal repair of ventral hernia in 1989. Prolene mesh is currently the most widely used because it is relatively inexpensive, easy to handle, has a memory (allows them to regain the original shape), and is firmly incorporated in the abdominal wall due to its ability to induce an intense inflammatory reaction. A 2 to 5 percent fistula rate has been reported with polypropylene mesh used intra-abdominally leading to the suggestion the great care must be taken to separate it from the bowel if it has to be used at all. However, some studies do not support this view. Bingener found no association of visceral adhesion when prolene was used with adequate omental interposition between it and the bowel. In another study involving 136 patients, Vrijland concluded that enterocutaneous fistula appears to be very rare after prolene mesh repair regardless of intraperitoneal placement, omental coverage or closing
the peritoneum.
A study comparing the biomaterials used in LVHR found polyester to have the highest incidence of infection, fistulization, and recurrence. The expanded polytetrafluoroethylene (ePTFE) has the longest history in the use for these hernias repair. The original description of the procedure used an early generation of the ePTFE product. The current product has one smooth surface with 3 microns ePTFE interstices, while the other side has 22 microns interstices to facilitate fibroblastic ingrowth for firm fixation. Other modifications of this product involve the incorporation of antimicrobials on the visceral surface. All of the composite prostheses have ePTFE and prolene or polyester but differ in the number and attachment of them together. There are no reports of intestinal fistulization or obstruction with ePTFE though it has also been found to induce inflammation and fibrosis in laboratory animals.
However, the use of synthetic materials is not without problems. As a foreign material, the repair site is subjected to inflammation, susceptibility to infection, and pain as a foreign body response. The encapsulation could affect the elastic function of the abdominal wall and the aesthetic outcome of the repair. This has stimulated the search for natural biological prostheses like surgisis, collagen, glycosaminoglycans from porcine intestinal submucosa, and alloderm. The financial cost to the clinical-benefit ratio for use of the substantially expensive composite meshes is unquantified and is likely to remain as such because, given the widespread acceptance of composite products, randomized, clinical comparison with prolene is unlikely to occur. Therefore, in selected circumstances, it may be acceptable to use a simple mesh, if this can be excluded from the bowel by tissue interposition be it omentum or peritoneum. A composite mesh should be considered as the current standard of care.
The extraperitoneal placement of the prostheses would in principle diminish the intra-abdominal complications associated with the formation of adhesions. It would also allow the safe use of conventional meshes like prolene, which has high intrinsic tensile strength, good memory, and cheaper. In addition, the peritoneal coverage over the entire mesh provides additional security of fixation and a better mechanical advantage. As such it can be seen as an advance over the onlay approach. However, the placement is technically demanding as evidenced by the high iatrogenic peritoneal tears in the largest series and it may not be feasible in the scarred abdomen of incisional and recurrent hernias, which constitute the bulk and seems to benefit most, from LVHR. Thus the issue of limitation of patient population amongst the technical feasibility and adequacy of defect coverage are issues of great concern before the method is accepted as an additional procedure for LVHR.
The good results and the attributed safety of LVHR are based on the large number of studies mainly utilizing the intraperitoneal approach. The generalization of the procedure has resulted in multiple variations of techniques. Overall, fewer complications are reported after LVHR than after open mesh repair especially in relation to wound and mesh infection. The efficacy of the inlay approach as an advancement of the conventional repair needs to be evaluated in terms of the several specific complications that are of particular relevance in laparoscopic procedures.
Bowel Injury
Probably the most dreaded complication is bowel injury and particularly if it is missed intraoperatively. It is a potentially lethal complication. The overall incidence of bowel injury does not differ significantly between open repair and laparoscopic repair and is generally low with either approach (1–5% when serosal injuries are included). Pneumoperitoneum may hinder the recognition of bowel injury at the time of operation. There have also been reports of late bowel perforation secondary to thermal injury with laparoscopic repair.
Minimizing the use of electrocauterization and ultrasonic dissection markedly reduces the risk of bowel injury. The visualization afforded by the pneumoperitoneum place adhesions between the abdominal wall and the bowel under tension. The high-intensity light source and the magnification inherent in the laparoscopy facilitate the identification of the least vascularized planes. As far as possible, direct grasping the bowel should be avoided preferring simply to push it or to grasp the adhesions themselves to provide counter traction. External pressure on the hernia may also help. Larger vessels in the omentum or adhesions are controlled with clips. Some degree of oozing from the dissected areas is tolerated; such oozing almost always settles down without specific hemostatic measures.
Adhesion
In cases of dense adhesions, it is preferable to divide the sac or the fascia rather than risk injury to the bowel. Densely adherent polypropylene mesh is best excised the abdominal wall rather than attempting to separate it from the serosa of the bowel. If bowel injury is suspected immediate and thorough inspection should be carried out. It may be difficult or impossible to find the exact site of injury later once the bowel has been released and freed of its attachments. Once the injury is recognized, it is the surgeon’s level of comfort with laparoscopic suture repair determines the best approach. With minimal spillage of bowel contents, the injury may be treated with either laparoscopic repair or open repair; the latter usually can be carried out through a mini-laparotomy over the injured area. Whether the mesh prosthesis is put primarily or later depends on the degree of contamination. More significant bowel injuries necessitate conversion to open repair. Missed injuries manifest postoperatively mandating re-exploration with occasional removal of the mesh and immediate recurrence of the hernia.
Infection
One of the greatest benefits of LVHR is the reduction in wound and mesh infections. In a detailed analysis of wound complications from a pooled data of forty-five published series involving 5340 patients, Pierce reported wound infection rates of 4.6 to 8 times to fold higher in open versus LVHR. The number of mesh infections was also significantly higher with open approaches. Wound problems are strongly linked with soft tissue dissection required for retro muscular placement of large pieces of mesh. The intraperitoneal approach obviates the need for this dissection that potentially devascularizes the fascia and causes hematoma formation, both of which contribute to infection. Although the incidence of mesh infection is very low the consequences are severe. Infections of prolene meshes can be managed locally with surgical drainage and excision of exposed, unincoporated segments but that of ePTFE requires removal in most cases due to its relatively low incorporation onto the body wall. Removal of the mesh results in a return of the defect and its added morbidity. An analysis of all series with more than 50 patients indicated a mesh infection rate of 0.6 percent, cellulitis of the trochar sites that resolved on antibiotics alone in 1.1 percent, and an overall wound and mesh complications of 1.7 percent. This has led to the widely perceived conclusion that the most compelling argument for LVHR is the minimization of soft tissue dissection and the associated reduction in the morbidity of local wound complications and potential infection of the implanted mesh. The high mesh infection rate reported in the inlay approach could be related to the extensive dissection of the peritoneal flap.
Seroma
Seroma formation is one of the most commonly reported complications in LVHR though it is not unique to laparoscopy. It swelling like mass occurs immediately after the operation in virtually all patients but disappears after a few weeks. Most seromas develop above the mesh and within the retained hernia sac. The mean incidence of seroma in reported series at a range of 4 to 8 weeks is 11.4 percent. In the largest multi-institutional trial, seromas that were clinically apparent more than 8 weeks were considered a complication and occurred in 2.6 percent. Regardless of whether they are aspirated under sterile conditions or allowed to resolve, they rarely cause long-term morbidity. Aspiration may increase the risk of mesh infection but is recommended if they enlarge or persist before they reach their extremes. Patients sometimes mistake a tense seroma for recurring incisional hernia, but appropriate preoperative discussion should provide them with significant reassurance on this point.
Although Feldman suggests that, seroma formation is not related to a particular type of mesh, Carbanjo and Heniford reported a higher incidence of seroma formation with ePTFE than prolene based meshes. The low incidence in the latter meshes has been attributed to the large pores of the prolene-based meshes that allow more efficient resorption of wound secretions into the abdominal cavity than ePTFE meshes. The dissection of the preperitoneal space during the inlay method may lead to more seroma formation. This is supported by the fact that, in the classical description of the onlay technique, it is emphasized that no attempts should be made to reduce or resect the hernia sac. This has been established to be unnecessary and to increase the incidence of seroma formation. The peritoneum interposed a barrier between the mesh and the abdominal cavity may hinder the direct drainage of this fluid regardless of the mesh used. Thus based on these facts, it seems plausible that the problem of seroma formation is expected to be higher in the inlay than the conventional onlay approach.
Postoperative Pain
After LVHR, about 5 percent of patients complain of persistent pain and point tenderness at the transabdominal suture site which usually resolves spontaneously within 6 to 8 weeks. If it does not, the injection of local anesthetic into the area around the painful suture has good results. Since missed enterotomy is a grave concern in LVHR, particularly after a difficult adhesiolysis, a correct interpretation of the significance of postoperative pain is an important issue. Whether or not to re-laparoscope a patient who experiences severe pain remains an important issue. A possible explanation of the common type of pain may be that the transabdominal suture entraps an intercostal nerve as it courses through the abdominal muscles. Local muscle ischemia may be another possibility. As such, it is an unavoidable adverse outcome of either approach so long as there is suture fixation of the prosthetic mesh. Whether it can be avoided by not using suture in the preperitoneal approach has to be weighed against the clinical benefit ratio of such a repair.
Obesity
The morbidly obese population represents a significant population of patients who present for ventral hernia repair. The advantages of minimal dissection, smaller wounds, and decreased wound complications using the onlay methods have been concluded in a recent review and all mitigate against the preperitoneal dissection of the inlay approach.
Recurrence
The ultimate measure of the effectiveness of hernia surgery is the recurrent rate. Recurrence rates after LVHR range from 1.1 to 13 percent whereas those after the open repairs ranged from 25 to 49 percent. In a multicenter series of 850 cases, the recurrence rate after a mean follow-up period of 20 months was 4.7 percent. The average recurrent rates using the onlay approach are approximately 4.2 percent although rates as high as 17 percent have been reported. The critical technical points related to recurrence are inadequate mesh fixation particularly with sutures and prostheses that overlap the defect by less than 2 to 3 cm. Other factors associated with high recurrent rates include postoperative complications, previous repairs, missed hernias as in the “Swiss cheese” defects, longer operating time, and obesity. The surgeon’s level of experience plays a significant role in patient outcome, as demonstrated by a group that compared the outcomes for their first 100 laparoscopic incisional hernia repair patients, with those for their second 100. Recurrence rates after a mean follow-up period of 36 months dropped from 9 percent in the first 100 patients to 4 percent in the second 100. In addition, the second set of patients were an average of 9 years older, had a higher percentage of recurrent hernias, and exhibited more comorbidities, yet despite these added challenges, operating time was not lengthened, length of stay was similarly short, and the complication rate was no different. A multivariate analysis of these variables indicated that prior failed hernia and increased estimated blood loss predicted recurrence while the other variables included; body mass index, defect size, and the size of the mesh did not have a positive correlation.
Although the results of large randomized trials are not available yet, the recent evidence to date suggests that the conventional onlay laparoscopic approach to the repair of ventral hernias is highly promising. The proposed laparoscopic preperitoneal placement of prostheses seems to negate most of the positive attributes of LVHR in most ways. This technique may be advantageous in small primary hernias, in a highly selected patients population. However, the widespread application of this approach or even the possibility of it being entered into a randomized trial appears dismal in the prevailing evidence and the patient's population that usually present with this structural disability.


