Laparoscopic Management of Stress Incontinence - Burch Suspension
Genuine stress incontinence is the involuntary loss of urine which occurs when the intravesical pressure exceeds the maximum urethral pressure in the absence of a detrusor contraction. The preferred therapy for genuine stress incontinence is surgery. Urinary incontinence is becoming more prevalent as the population ages. A significant improvement in the psychological status of these patients after the successful surgical cure of stress incontinence has been demonstrated.
Pathophysiology
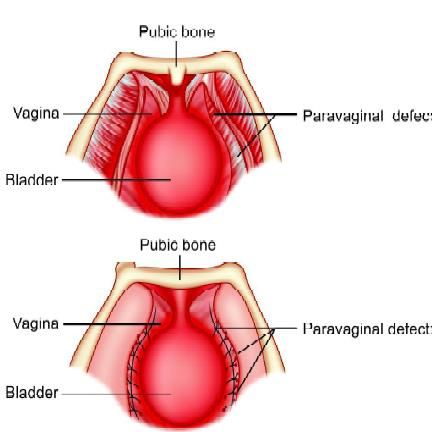
In genuine stress incontinence, the proximal urethra is displaced outside the abdominal cavity. Stress incontinence then results from inadequate transmission of increases in intra-abdominal pressure to the proximal urethra. The urethra has in fact lost its retropubic position due to attenuated support. As a result, coughing will produce an immediate increase in intravesical pressure but not a concomitant increase in intraurethral pressure, and a dribble of urine results. More than 160 types of operations are available to correct stress urinary incontinence, an optimal approach has not been developed. The Burch procedure is considered by many to be the gold standard for surgical treatment of genuine stress incontinence. The Burch procedure requires the elevation of the anterior wall of the vagina to the level of the origin of the paravaginal fascia by suspension from Cooper’s ligaments (iliopectineal ligaments). A properly performed Burch procedure cures 93 percent.
Laparoscopic Burch suspension
Laparoscopic Anatomy
The space of Retzius lies between the vesicoumbilical fascia posteriorly and the posterior rectus sheath and pubic bone, anteriorly. This is the space first entered in Burch suspension.
Preoperative Evaluation
Postmenopausal women should receive at least 3 months of estrogen replacement therapy. Preoperative evaluation includes history, physical, gynecologic, and neurologic examinations. The routine tests include a stress test, a Q-tip test, urinalysis, urine culture, and sensitivity. All women should undergo a urodynamic evaluation, with an emphasis on voiding time, voiding volume, and post-void residual urine volume. Stress incontinence is diagnosed by a positive stress test in the absence of simultaneous detrusor contractions or pressure equalization on the stress urethral closure pressure profile.
Operative Technique
Following the induction of general endotracheal anesthesia, a Foleys catheter should be applied in the bladder. A 10 mm operative video laparoscope is inserted infraumbilical and three 5 mm accessory trocars are inserted according to the baseball diamond concept. 300 cc of methylene blue solution is placed into the bladder to keep the bladder weighted down and to allow the clear delineation of the dome of the bladder. Three port techniques are used one in the umbilicus and two 5 cm lateral and slightly below the umbilicus. The anterior abdominal wall peritoneum 3 to 5 cm above the symphysis pubis is cut and pulled down. To enter the space of Retzius intra-abdominally, the umbilical ligaments are identified laterally.
The space of Retzius is developed using blunt and CO2 dissection of fibrofatty tissue. Care is taken to avoid obturator nerve and aberrant obturator vessel injury.
The midline trocar entry and anatomic landmarks, including the round ligament from the internal ring, are used to avoid bladder injury. Blunt dissection, hydrodissection, and the CO2 laser for sharp dissection are used to expose the retropubic space. A surgeon should stay close to the back of the pubic bone, dropping the anterior bladder wall, vaginal wall, and urethra downward. Dissection is limited over the urethra in the mid-line, to approximately 2 cm lateral to the urethra to protect its delicate musculature. An assistant should introduce one finger on each side of the catheterized urethra, elevating the lateral vaginal fornix. The overlying fibrofatty tissue is cleared laparoscopically from the anterior vaginal wall. The bladder is dissected medially from the paravaginal fascia from lateral to medial. Blunt dissection with atraumatic grasper is continued until the urethrovesical junction becomes apparent and the white glistening tissue of the paravaginal fascia appears.
Bladder muscle fibers of the urethrovesical junction should not be damaged at the time of dissection. Bleeding if happens in this area is controlled only with bipolar electrocoagulation because; monopolar current can cause perforation of bladder or stricture of the ureter. Retropubic dissection is continued until Cooper’s ligament is clearly exposed. Dissection in the space of Retzius is difficult in patients who have undergone previous laparotomy. Pneumoperitoneum pressure provides exposure to space and its contents out to the obturator nerve.
The space of Retzius also can be entered with a preperitoneal approach just like transabdominal preperitoneal hernia surgery. The balloon dissector consists of a cannula, balloon system. The dissector is inserted through a 10 mm infraumbilical incision. It is advanced between the rectus muscle and the anterior surface of the posterior rectus sheath towards the symphysis pubis. The dissector’s external sheath is removed, and the balloon is inflated with approximately 500 ml of saline solution. During inflation, the balloon unrolls sideways and exerts a perpendicular force that separates tissue layers. Blunt dissection of the connective tissues is propagated as the balloon expands. Full dissection takes about one minute but the surgeon should keep the balloon inflated for 5 minutes to achieve proper hemostasis. The lateral pressure of the balloon will stop capillary bleeding if kept for 5 minutes. When maximal volume is reached, the balloon is deflated and removed through the incision.
The dissected space is insufflated with CO2 at a pressure of 8 to 10 mm Hg. The predefined shape of the balloon, its non-elastomeric material, and the incompressible character of the saline assure a large, relatively bloodless working space of predictable size and shape. The space is adequate for the identification of pertinent landmarks and unencumbered manipulation of endoscopic surgical instruments.
After complete dissection, the paravaginal fascia is identified as glistening fascia over the finger of an assistant. Using a needle holder a suture placed over the paravaginal fascia at the level of the urethrovesical junction, approximately 1 to 1.5 cm from the urethra. The urethrovesical junction can be identified easily because of the Foley bulb with the catheter under gentle traction. This suture is placed perpendicular to the vaginal axis to include approximately 0.5 to 1 cm of tissue. The complete vaginal fascia should be taken so that it will not cut through. Precaution should be taken that suture does not penetrate the vaginal mucosa. Once the suture is placed over the paravaginal fascia, the suture is fixed to Cooper’s ligament or the midline of the symphysis pubis. The suture is tied either intracorporeal or extracorporeally with the help of an assistant who lifts the vagina upward and forward. The urethra is observed carefully to prevent it from being compressed against the pubic bone. This same suturing technique is repeated on the opposite side, the aim is to create a platform on which the bladder neck can rest. If the suspension is not enough with one suture, a second and rarely, the third set of sutures can be placed along the base of the bladder proximally.
After taking suture on both sides, cystoscopy should be performed to ensure that there is no suture pierced in the bladder, cystoscopy also helps to assess the urethrovesical junction angle, and to check ureteral patency.
Proline mesh can be used instead of a direct suture for bladder neck suspension. Medially over paravaginal fascia, the mesh is fixed by sutures, and laterally over Cooper’s ligament, it should be fixed with the help of Protak or Anchor. One of the advantages of mesh is that after some time it gives a very good platform for the bladder neck due to fibrosis developed through the mesh. Once the procedure is finished the pneumoperitoneum pressure is decreased and the retropubic space is evaluated. If there is any bleeding, it is controlled with bipolar electrocoagulation. The peritoneal defect may be left open to heal spontaneously, or, if it is large, it should be closed laparoscopically with three to four interrupted or purse-string sutures. The laparoscope is withdrawn from the abdomen and the procedure concluded. The Foley’s catheter should be left in place for 2 to 3 days. If there is any fear of detrusor instability or bladder injury, the Foley’s should be in place for one week or more.
Intercourse should be avoided for 6 to 8 weeks postoperatively and the patients are advised to avoid heavy lifting or strenuous exercise for at least 2 months.
Genuine stress incontinence is the involuntary loss of urine which occurs when the intravesical pressure exceeds the maximum urethral pressure in the absence of a detrusor contraction. The preferred therapy for genuine stress incontinence is surgery. Urinary incontinence is becoming more prevalent as the population ages. A significant improvement in the psychological status of these patients after the successful surgical cure of stress incontinence has been demonstrated.
Pathophysiology
In genuine stress incontinence, the proximal urethra is displaced outside the abdominal cavity. Stress incontinence then results from inadequate transmission of increases in intra-abdominal pressure to the proximal urethra. The urethra has in fact lost its retropubic position due to attenuated support. As a result, coughing will produce an immediate increase in intravesical pressure but not a concomitant increase in intraurethral pressure, and a dribble of urine results. More than 160 types of operations are available to correct stress urinary incontinence, an optimal approach has not been developed. The Burch procedure is considered by many to be the gold standard for surgical treatment of genuine stress incontinence. The Burch procedure requires the elevation of the anterior wall of the vagina to the level of the origin of the paravaginal fascia by suspension from Cooper’s ligaments (iliopectineal ligaments). A properly performed Burch procedure cures 93 percent.

Laparoscopic Burch suspension
Laparoscopic Anatomy
The space of Retzius lies between the vesicoumbilical fascia posteriorly and the posterior rectus sheath and pubic bone, anteriorly. This is the space first entered in Burch suspension.
Preoperative Evaluation
Postmenopausal women should receive at least 3 months of estrogen replacement therapy. Preoperative evaluation includes history, physical, gynecologic, and neurologic examinations. The routine tests include a stress test, a Q-tip test, urinalysis, urine culture, and sensitivity. All women should undergo a urodynamic evaluation, with an emphasis on voiding time, voiding volume, and post-void residual urine volume. Stress incontinence is diagnosed by a positive stress test in the absence of simultaneous detrusor contractions or pressure equalization on the stress urethral closure pressure profile.
Operative Technique
Following the induction of general endotracheal anesthesia, a Foleys catheter should be applied in the bladder. A 10 mm operative video laparoscope is inserted infraumbilical and three 5 mm accessory trocars are inserted according to the baseball diamond concept. 300 cc of methylene blue solution is placed into the bladder to keep the bladder weighted down and to allow the clear delineation of the dome of the bladder. Three port techniques are used one in the umbilicus and two 5 cm lateral and slightly below the umbilicus. The anterior abdominal wall peritoneum 3 to 5 cm above the symphysis pubis is cut and pulled down. To enter the space of Retzius intra-abdominally, the umbilical ligaments are identified laterally.
The space of Retzius is developed using blunt and CO2 dissection of fibrofatty tissue. Care is taken to avoid obturator nerve and aberrant obturator vessel injury.
The midline trocar entry and anatomic landmarks, including the round ligament from the internal ring, are used to avoid bladder injury. Blunt dissection, hydrodissection, and the CO2 laser for sharp dissection are used to expose the retropubic space. A surgeon should stay close to the back of the pubic bone, dropping the anterior bladder wall, vaginal wall, and urethra downward. Dissection is limited over the urethra in the mid-line, to approximately 2 cm lateral to the urethra to protect its delicate musculature. An assistant should introduce one finger on each side of the catheterized urethra, elevating the lateral vaginal fornix. The overlying fibrofatty tissue is cleared laparoscopically from the anterior vaginal wall. The bladder is dissected medially from the paravaginal fascia from lateral to medial. Blunt dissection with atraumatic grasper is continued until the urethrovesical junction becomes apparent and the white glistening tissue of the paravaginal fascia appears.
Bladder muscle fibers of the urethrovesical junction should not be damaged at the time of dissection. Bleeding if happens in this area is controlled only with bipolar electrocoagulation because; monopolar current can cause perforation of bladder or stricture of the ureter. Retropubic dissection is continued until Cooper’s ligament is clearly exposed. Dissection in the space of Retzius is difficult in patients who have undergone previous laparotomy. Pneumoperitoneum pressure provides exposure to space and its contents out to the obturator nerve.
The space of Retzius also can be entered with a preperitoneal approach just like transabdominal preperitoneal hernia surgery. The balloon dissector consists of a cannula, balloon system. The dissector is inserted through a 10 mm infraumbilical incision. It is advanced between the rectus muscle and the anterior surface of the posterior rectus sheath towards the symphysis pubis. The dissector’s external sheath is removed, and the balloon is inflated with approximately 500 ml of saline solution. During inflation, the balloon unrolls sideways and exerts a perpendicular force that separates tissue layers. Blunt dissection of the connective tissues is propagated as the balloon expands. Full dissection takes about one minute but the surgeon should keep the balloon inflated for 5 minutes to achieve proper hemostasis. The lateral pressure of the balloon will stop capillary bleeding if kept for 5 minutes. When maximal volume is reached, the balloon is deflated and removed through the incision.
The dissected space is insufflated with CO2 at a pressure of 8 to 10 mm Hg. The predefined shape of the balloon, its non-elastomeric material, and the incompressible character of the saline assure a large, relatively bloodless working space of predictable size and shape. The space is adequate for the identification of pertinent landmarks and unencumbered manipulation of endoscopic surgical instruments.
After complete dissection, the paravaginal fascia is identified as glistening fascia over the finger of an assistant. Using a needle holder a suture placed over the paravaginal fascia at the level of the urethrovesical junction, approximately 1 to 1.5 cm from the urethra. The urethrovesical junction can be identified easily because of the Foley bulb with the catheter under gentle traction. This suture is placed perpendicular to the vaginal axis to include approximately 0.5 to 1 cm of tissue. The complete vaginal fascia should be taken so that it will not cut through. Precaution should be taken that suture does not penetrate the vaginal mucosa. Once the suture is placed over the paravaginal fascia, the suture is fixed to Cooper’s ligament or the midline of the symphysis pubis. The suture is tied either intracorporeal or extracorporeally with the help of an assistant who lifts the vagina upward and forward. The urethra is observed carefully to prevent it from being compressed against the pubic bone. This same suturing technique is repeated on the opposite side, the aim is to create a platform on which the bladder neck can rest. If the suspension is not enough with one suture, a second and rarely, the third set of sutures can be placed along the base of the bladder proximally.
After taking suture on both sides, cystoscopy should be performed to ensure that there is no suture pierced in the bladder, cystoscopy also helps to assess the urethrovesical junction angle, and to check ureteral patency.
Proline mesh can be used instead of a direct suture for bladder neck suspension. Medially over paravaginal fascia, the mesh is fixed by sutures, and laterally over Cooper’s ligament, it should be fixed with the help of Protak or Anchor. One of the advantages of mesh is that after some time it gives a very good platform for the bladder neck due to fibrosis developed through the mesh. Once the procedure is finished the pneumoperitoneum pressure is decreased and the retropubic space is evaluated. If there is any bleeding, it is controlled with bipolar electrocoagulation. The peritoneal defect may be left open to heal spontaneously, or, if it is large, it should be closed laparoscopically with three to four interrupted or purse-string sutures. The laparoscope is withdrawn from the abdomen and the procedure concluded. The Foley’s catheter should be left in place for 2 to 3 days. If there is any fear of detrusor instability or bladder injury, the Foley’s should be in place for one week or more.
Intercourse should be avoided for 6 to 8 weeks postoperatively and the patients are advised to avoid heavy lifting or strenuous exercise for at least 2 months.






