Laparoscopic Management of Pancreatic Pseudocyst
Pancreatic pseudocysts may be defined as a collection of pancreatic secretions, serous fluid, or necrotic debris surrounded by a nonepithelialized wall made up of granulation tissue and a variable degree of fibrous tissue. Pancreatic pseudocysts must be distinguished from true cysts of the pancreas, which are characterized histologically by the presence of an epithelial lining. Pseudocyst formation is the result of a post-inflammatory process arising from patients with acute or chronic pancreatitis. An understanding of the natural history of pancreatic pseudocyst is important when deciding on invasive therapy versus expectant management. Studies like those by Bradley and coworkers had a great influence on the management of pseudocystic disease. Bradley and coworkers suggested the likelihood of regression diminished and the likelihood of complications rose dramatically after a 6 weeks period. More recent data suggest that this patient population may be watched safely for longer periods. Yeo and coworkers followed asymptomatic patients with pseudocysts by CT scanning for 1 year (48% were successfully observed with only a 2.7% complication rate). The only predictor for intervention was size greater than 7.4 + /–0.6 cm. General asymptomatic patients with pancreatic pseudocyst may be followed up for extended periods of time. This conservative approach is more likely to be successful in patients with small 6 cm pseudocysts. Other options are available for drainage procedures (e.g. percutaneous transgastric, ERCP).
Laparoscopic Pseudocyst Drainage
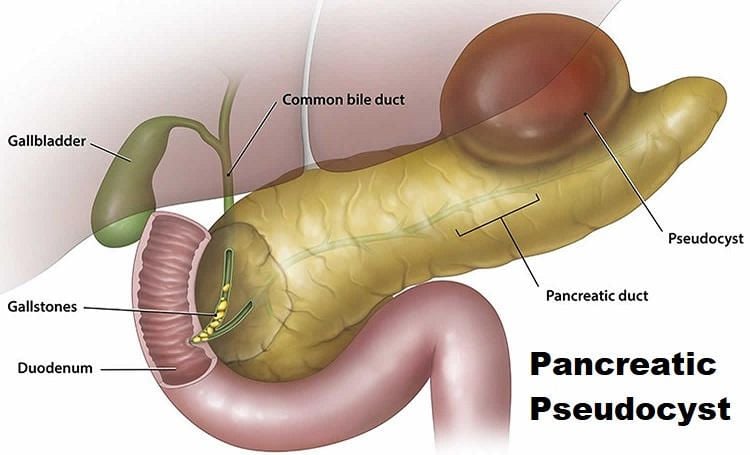
Preoperative decision making and subsequent laparoscopic operative approach should mimic that of open operative planning. The selection of procedure will depend on the anatomic location of the pseudocyst, pseudocyst size, and associated pancreatic duct or distal common bile duct abnormalities. Reports by Newell and coworkers document that pseudocyst-gastrostomy is technically easier than pseudocyst-jejunostomy while remaining equally efficacious. Laparoscopic pseudocyst-gastrostomy is technically easier, but cyst-jejunostomy is also technically feasible for the cyst not amenable to gastric drainage by standard surgical principles.
Laparoscopic pseudocyst-gastrostomy was first performed by Petelin in 1991. Principles of operative drainage include biopsy of the cyst wall to rule out neoplasm, dependent drainage, and precise hemostatic technique to avoid hemorrhage. The patient position and port placement are the same as described for the bypass procedure. The pseudocyst may often be seen pushing the stomach forward. A small gastrotomy is established with cautery over the most prominent portion of the pseudocyst. Ultrasound may be helpful in locating the pseudocyst and the site of the initial gastrotomy.
The gastrotomy is then extended for several centimeters with electrocautery. A small window is developed through the posterior wall of the stomach with electrocautery. One must remember that the posterior wall of the stomach and cyst capsule will be fused and that this requires a deeper dissection with cautery than felt comfortable by the surgeon. Ultrasound may be helpful to plan dissection where the stomach wall or cyst is thinnest. The window is made large enough to accommodate the endoscopic stapler. A biopsy of the wall may be carried out at this time. Two firings of the stapler are used to create a substantial anastomosis (stapler insertion through the more comfortable 12 mm pon, usually the right). Hemostasis at the staple line should be assured. The gastrotomy is closed with either sutures or staples.
Conclusion
The laparoscopic approach to hepatic and pancreatic surgery has rapidly been shown to be of considerable value. The laparoscopic approach to the pancreas has value with respect to staging, bypass procedures, and pseudocyst drainage. Pancreatic resection is feasible, but must still be considered investigational.
Pancreatic pseudocysts may be defined as a collection of pancreatic secretions, serous fluid, or necrotic debris surrounded by a nonepithelialized wall made up of granulation tissue and a variable degree of fibrous tissue. Pancreatic pseudocysts must be distinguished from true cysts of the pancreas, which are characterized histologically by the presence of an epithelial lining. Pseudocyst formation is the result of a post-inflammatory process arising from patients with acute or chronic pancreatitis. An understanding of the natural history of pancreatic pseudocyst is important when deciding on invasive therapy versus expectant management. Studies like those by Bradley and coworkers had a great influence on the management of pseudocystic disease. Bradley and coworkers suggested the likelihood of regression diminished and the likelihood of complications rose dramatically after a 6 weeks period. More recent data suggest that this patient population may be watched safely for longer periods. Yeo and coworkers followed asymptomatic patients with pseudocysts by CT scanning for 1 year (48% were successfully observed with only a 2.7% complication rate). The only predictor for intervention was size greater than 7.4 + /–0.6 cm. General asymptomatic patients with pancreatic pseudocyst may be followed up for extended periods of time. This conservative approach is more likely to be successful in patients with small 6 cm pseudocysts. Other options are available for drainage procedures (e.g. percutaneous transgastric, ERCP).
Laparoscopic Pseudocyst Drainage

Preoperative decision making and subsequent laparoscopic operative approach should mimic that of open operative planning. The selection of procedure will depend on the anatomic location of the pseudocyst, pseudocyst size, and associated pancreatic duct or distal common bile duct abnormalities. Reports by Newell and coworkers document that pseudocyst-gastrostomy is technically easier than pseudocyst-jejunostomy while remaining equally efficacious. Laparoscopic pseudocyst-gastrostomy is technically easier, but cyst-jejunostomy is also technically feasible for the cyst not amenable to gastric drainage by standard surgical principles.
Laparoscopic pseudocyst-gastrostomy was first performed by Petelin in 1991. Principles of operative drainage include biopsy of the cyst wall to rule out neoplasm, dependent drainage, and precise hemostatic technique to avoid hemorrhage. The patient position and port placement are the same as described for the bypass procedure. The pseudocyst may often be seen pushing the stomach forward. A small gastrotomy is established with cautery over the most prominent portion of the pseudocyst. Ultrasound may be helpful in locating the pseudocyst and the site of the initial gastrotomy.
The gastrotomy is then extended for several centimeters with electrocautery. A small window is developed through the posterior wall of the stomach with electrocautery. One must remember that the posterior wall of the stomach and cyst capsule will be fused and that this requires a deeper dissection with cautery than felt comfortable by the surgeon. Ultrasound may be helpful to plan dissection where the stomach wall or cyst is thinnest. The window is made large enough to accommodate the endoscopic stapler. A biopsy of the wall may be carried out at this time. Two firings of the stapler are used to create a substantial anastomosis (stapler insertion through the more comfortable 12 mm pon, usually the right). Hemostasis at the staple line should be assured. The gastrotomy is closed with either sutures or staples.
Conclusion
The laparoscopic approach to hepatic and pancreatic surgery has rapidly been shown to be of considerable value. The laparoscopic approach to the pancreas has value with respect to staging, bypass procedures, and pseudocyst drainage. Pancreatic resection is feasible, but must still be considered investigational.


