Today is Sunday. The day to learn laparoscopic G.I anastomosis for our online trainee members.
How much has the introduction of laparoscopic surgery changed open surgery?
Advances in miniaturisation, camera technology, video presentation and surgical instrumentation from the late 1980s onwards allowed the abdominal surgeon to operate laparoscopically, with a good view and with instruments in both hands. Since 1990, laparoscopic cholecystectomy has been introduced throughout the Western world,1 in a rapid and uncontrolled fashion. Evidence-based medicine played little role in this rapid dissemination of a new technique. Surgeons, industry, patients and the media drove the expansion. Fortunately, this operation has proven to be reliable, effective and reasonably safe.1,2
By 2000, most of the alimentary system as well as solid organs and glands had been removed laparoscopically or endoscopically, across most surgical specialties.3 A large number of purely functional operations have also been performed. The threshold for some surgical interventions has been lowered because of the perception that laparoscopic surgery is less painful than open surgery and recovery quicker.4
Many surgical groups have now turned their attention from the question “Can it be done?” to wondering “Should it be done?” Doubts have arisen because of issues such as the lengthy operative time required for some procedures and the cost of specialised instrumentation. Furthermore, the use of laparoscopic surgery in cancer patients has been dogged by concern about possible port-site recurrences.5 There is, however, mounting evidence that this is not a significant issue.6
The question of what should be done has led to several international trials of open versus laparoscopic surgery. In the USA the Clinical Outcomes of Surgical Therapy (COST) study is comparing outcomes of open surgery with those of laparoscopic surgery for colon cancer. Similar trials are underway in the United Kingdom, with the Conventional versus Laparoscopic Assisted Surgery in Colorectal Cancer (CLASSIC) trial, and Australia and New Zealand, with the Australasian laparoscopic colon cancer (ALCCaS) trial. These trials are not just looking at survival rates but also issues such as day stay, cost benefit and quality of life. These trials have also led us to re-evaluate open surgery, which has often been led by expert opinion and dogma rather than evidence-based management decisions.
Minimally invasive surgery has taken much of the credit for the reduction in hospital stay, reduced morbidity and earlier return to work in elective surgical patients over the last ten years. However, many of these benefits are now seen in open surgery cases. Many aspects of peri-operative surgical care have changed and laparoscopic surgery has worked as a catalyst for these changes rather than as an autonomous influence.
The significance of the role that laparoscopic surgery has played in recent changes in surgical practice can be illuminated by posing the following three questions:
- Is laparoscopic surgery really different from open surgery?
- Did the laparoscopic revolution occur during a period of parallel peri-operative revolution?
- Was laparoscopic surgery a catalyst to change in open surgery?
1. Is laparoscopic surgery really different from open surgery?
The perceived advantages of laparoscopic cholecystectomy over conventional laparotomy are shorter hospital stay, earlier return of bowel motility, less post-operative pain, better cosmesis, earlier return to normal activity, earlier return to work and hence less total cost to society, and fewer long-term complications associated with adhesions. These perceptions have been confirmed by some, but not all, randomised controlled trials.7,–9 Similar claims that were made for laparoscopic appendicectomy10,11 and laparoscopic hernia repair12 have been even harder to prove. However, in many countries and with many operations, the opportunity to undertake prospective, comparative studies is now very limited due to consumer demand and expectations.
2. Did the laparoscopic revolution occur during a period of parallel peri-operative revolution?
Anaesthesia and analgesia There have been significant changes in anaesthetic practice with the introduction of short-acting, fast-emerging agents such as propofol, fentanyl and sevoflurane. Patients are recovering from their general anaesthetic faster and with less nausea and vomiting. The laryngeal mask airway, developed in 1983, has decreased irritation and trauma to the airway,13 and has reduced induction times and recovery times by removing the need for paralysis.13,14 Anaesthetic monitoring and safety have also improved considerably. Regional anaesthesia has become widespread and has reduced the morbidity associated with many operations.15 Epidural anaesthesia can reduce post-operative ileus and decrease post-operative pain.16,17 The patient-controlled analgesia (PCA) device has also increased the ability of the patient to self-regulate pain.18
The use of nonsteroidal anti-inflammatory drugs has decreased opioid requirements, and in turn has reduced post-operative nausea and vomiting.19 Early return to full awareness and effective post-operative pain control allow more rapid mobilisation and this has led to a reduction in both respiratory and thromboembolic complications.20 Newer antiemetics such as ondansetron21 and the use of steroids as antiemetics have been added to standard regimes with increased effectiveness.
Case management Day-of-admission surgery has become the standard of practice in many institutions.22 It effectively removes a day from the length of stay data. This needs to considered when comparing laparoscopy with historical open series. True day surgery (rather than 23-hour stay) is routine for many conditions and is increasingly used for major surgery such as laparoscopic cholecystectomy,22–24 and laparoscopic fundoplication.
Nurses and other healthcare professionals have increased their scope of practice. Nurses are tertiary graduates and have become increasingly specialised.25 care nurses, enterostomal therapists, diabetic nurses, and palliative care nurses are found in most major hospitals.
The drive for efficiency The increasing appetite for healthcare expenditure and the limited budget for such expenditure has made rationing of healthcare inevitable.26 Evidence-based medicine is being used by health providers to look dispassionately for the best combination of quality and cost containment. Clinical pathways, practice guidelines and protocols have become commonplace with the aim to increase efficiency and quality of care.27–30 When there is clear evidence for a superior technique, it can be introduced rapidly. Patients also have access to an abundance of information from the Internet. They are, therefore, usually better able to actively contribute to the diagnostic and interventional decisions taken during their illness.
Conservative post-operative attitudes have changed. Many patients are mobilised, fed and discharged sooner as the dogma evaporates. The normal expectation in most hospitals, for example, is that patients go home the day following appendicectomy. The physiotherapist is involved with all ‘at risk’ surgical patients.31 Earlier discharge to the community involves pre-operative discharge planning, and the involvement of social workers, occupational therapists and community nurses. The presence of post-operative rehabilitation wards may also contribute to shorter stays on the surgical ward.
3. Was laparoscopic surgery a catalyst to change in open surgery?
Without doubt, laparoscopic surgery has been a catalyst for change in many technical aspects of open surgery. The confidence to perform operations through small incisions, to mobilise, feed and discharge open surgery patients earlier, and the way we undertake operations have all changed because of the experience of laparoscopic surgery.
Hernia repair provides a good example of this. Until the early 1990s a sutured Shouldice or Bassini groin hernia repair was normal practice. Level one evidence has since demonstrated that the tension-free Lichtenstein mesh repair is the new gold standard for open surgery.32,33 Differences in outcomes between the open mesh repair and laparoscopic mesh repair are subtle, but both are superior to sutured open repair.32,34,35 It is feasible that the rapid introduction of laparoscopic mesh repair accelerated the acceptance of mesh repair in general, and encouraged the non-laparoscopic surgeon to rapidly adopt the best open repair possible.
Patients having laparoscopic colon resections are fed sooner and discharged earlier, often without waiting for a formed bowel motion. The confidence that it is safe to do this has been acquired from laparoscopic resections and is now applied to open resections. There are reports of patients who have had open colectomies being discharged from hospital after two days where the operation has been undertaken through small incisions, and the patient has had peri-operative, multimodal, intensive rehabilitation.36
Summary
There have been significant advances in the operative and peri-operative care of surgical patients during the ‘laparoscopic decade’. The technical aspects of laparoscopy have taken a lot of the credit for this. It may be, however, that properly constructed randomised trials would now show only a small difference in outcome between open surgery and laparoscopic surgery for some conditions. This could be due to parallel advances in anaesthesia and peri-operative management, and technical developments in open surgery. Laparoscopic surgery may well have been the catalyst for many of these improvements.
Laparoscopic and hand assisted GI anastomosis
Limitations of traditional laparoscopic surgery
The major limitation of have been the following:
- The human hand is a wonderful structure and provides multitude of different functions during open surgery. This function is absent during standard laparoscopic surgery since the abdomen is closed and the procedure is performed with long surgical instruments inserted from the outside into the abdomen.
- Two dimensional image of the laparoscope: The image transmitted by the laparoscopic camera that surgeon utilizes as his eyes is a two dimensional image. For some procedures this is a major limitation because of the poor depth perception that is associated with two dimensional images.
- Retraction of internal organs: During open surgery insertion of the hand into the abdomen allows the surgeon to move the intestine and other organs away from the site of the surgery. During standard laparoscopic surgery the hand is not introduced into the abdomen and introducing long thin instruments into the abdomen performs the surgery. Retraction of internal organs is often a major problem for some procedures.
- Limitation of instruments: the standard instruments in laparoscopic surgery are long thin instruments. These instruments are poorly suited for many complex laparoscopic procedures.
New technologies for advance laparoscopic surgery
Development of new instruments and innovation in technique is required for widespread use of advanced laparoscopy for the treatment of abdominal conditions. New technologies may overcome many of the limitations (noted above) of standard laparoscopic techniques. We have utilized the new technologies to develop laparoscopic operations for the pancreas, liver and the bile duct.
Two new technologies that are particularly promising are: hand access devices and robotic surgery.
Hand access devices
The human hand performs many functions during surgery that are difficult to reproduce with laparoscopic instruments. The loss of the ability to place the hand into the abdomen during traditional laparoscopic surgery has limited the use of laparoscopy for complex abdominal surgery on the pancreas, liver and bile duct.
New laparoscopic hand-access devices that allows the surgeon to place a hand into the abdomen during laparoscopic surgery and perform many of the different functions with the hand that were previously possible only during open surgery. We have utilized this new device to develop a variety of laparoscopic pancreatic, liver and biliary procedures such as the Whipple operation, distal pancreatectomy and liver resection that were not possible previously by standard laparoscopic techniques.
Robot-assisted surgery utilizing the Da Vinci computer robot system
Da Vinci™ is a computer-assisted robotic system that expands a surgeon's capability to operate within the abdomen in a less invasive way during laparoscopic surgery. Da Vinci™ system allows greater precision and better visualization compared to standard laparoscopic surgery.
The USC University Hospital is the first hospital in Southern California to perform robotically-assisted surgery using the da Vinci™ Surgical System.
The operations with the Da Vinci System are performed with no direct mechanical connection between the surgeon and the patient. The surgeon is remote from the patient, working a few feet from the operating table while seated at a computer console with a three-dimensional view of the operating field. The physician operates two masters (similar to joysticks) that control the two mechanical arms on the robot. The mechanical arms are armed with specialized instruments with hand-like movements which carry out the surgery through tiny holes in the patient’s abdomen. The arms eliminate any hand tremor by the surgeon and offer motion scaling – allowing extremely precise movements within the patient.
We are presently exploring the role of this new technology for complex operations on the pancreas, bile duct and the liver.
Some general points about anastomosis
It is important to take time to positively identify
- The optimum location of the enterotomy, in terms of anatomical position (distance distally).
- Position with regard to identification of the antimesenteric border.
- Orientated in longitudinal axis
- Avoid both tension, and unnecessary redundancy.
- Ensure direction of flow (anteperistaltisc)
- Remember the differential thickness of bowel wall.
- The anastomosis should be adequate (not too small and not too large).
- Haemostasis on enterotomy
- Security of closure, and prevention of anastomotic leakage.
Stapled anastomosis
The use of disposable stapling guns has simplified a number of endoscopic procedures such as the division of vascular pedicles and gut anastomosis.
The equipment we use in World Laparoscopy Hospital is that made by Tyco. There are other manufacturers. The Tyco staplers are of the following types:
- Circular different sizes
- Linear cutting different stapling lengths and staple sizes, reloadable cartridges with different staple size
- Linear non-cutting
- Deflecting cutting roticulator
The equipment will be demonstrated and the technique for a side to side anastomosis outlined. The following important points are emphasized:
- Port positions for stapling
- Stay sutures for tensioning
- Enterotomy positioning and size
- Positioning and angulation of the instrument prior to closure
- Checking suture line
- Complete closure of residual opening
End to end anastomosis can also be carried out by stapling closed bowel ends side by side.
Clinical Applications
An anterior or posterior, side to side anastomosis of stomach and jejunum done laparoscopically can be a satisfactory palliative procedure and is just one example of the use of this technique. Likewise a laparoscopic cholecystojejunostomy may to relieve jaundice and itching in patients with inoperable pancreatic cancer may be performed by stapling.
Sutured anastomosis
Sutured anastomosis can be carried out endoscopically, although the process is demanding in terms of skill and time. We use this as a task to allow you to assess the progress that you have made over the week and hopefully to give you confidence in your new abilities. However it is pertinent to note that staplers may not always be available, or appropriate, and even if a stapler is used, you require the skills to perform a sutured closure if the stapled anastomosis is not perfect.
Important points to remember are:
- Port positioning
- Use of and communications with your assistant
- Positioning of sutures, especially at the corners
- Spacing the sutures (remember the magnification)
- Tensioning of sutures
Direction of suturing
It is important that you suture at the right height — ideally your elbows should be held adducted and at right angles. Keep you wrists loose and remember that you have two hands that must manipulate to help each other. The choreography is as follows:
- The suturing line is started with a ‘starter knot’ (surgeons or tumbled square knot).
- The two needle holders must be kept in view and used in concert with each other.
- Passage from right to left through the tissue edges (bites consisting of entry an exit points) with dominant needle holder.
- The needle is picked up from the exit point by the passive needle holder (NH).
- It is transferred to dominant needle holder for the next bite if the orientation is correct. Otherwise it is dropped and re-orientated in the needle holder. Once the suture has passed through the two edges, the thread is pulled trough, handing the suture one needle holder to the other.
- The distance between the suture bites must be approximately equal to the depth of the bites.
INTRACORPOREAL KNOT TYING WITH THE ENDO STITCH
The Stringer Laparoscopic Suturing Technique
Intracorporeal knot tying utilizing the Endo Stitch is fast and easy and does not require a knot pusher.
To create an intracorporeal knot at the end of the suture line, place the needle in the left jaw position and pass it under the suture line at the end of the incision (Figure 3A). Toggle the needle to the right jaw to create a loop.
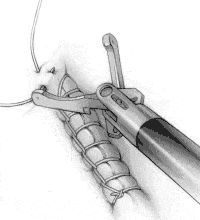
FIGURE 3A
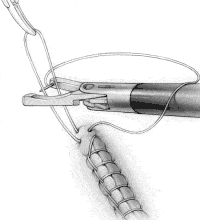
FIGURE 3B
Have the surgical assistant grasp the loop and pull it vertically (Figure 3B). With the needle in the right jaw, place the loop of suture that the assistant is holding between the jaws of the Endo Stitch.
Toggle the needle to the left jaw and pull the needle around the loop held by the assistant, passing under the suture on the right side (Figure 3C). This creates a flat surgical knot.
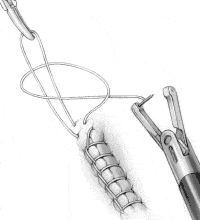
FIGURE 3C
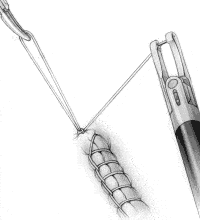
FIGURE 3D
Tighten the knot by having the surgical assistant pull in one direction while the surgeon pulls in the opposite direction with the Endo Stitch in the closed position (Figure 3D). Repeat the steps shown in Figures 3B, 3C and 3D to create the second tie in the knot. Additional ties may also be placed, as desired by the surgeon, to totally secure the knot.
LAVH
An Innovative New Technique Hysterectomy (surgical removal of the uterus) is one of the most commonly performed procedures in the United States. Abdominal hysterectomy typically requires women to spend four to six days in the hospital and four to six weeks recuperating at home.
Now a technique called laparoscopy makes it possible to perform this procedure in a minimally invasive manner, allowing patients to go home one or two days after surgery and to resume full activity within a week or two.
Hysterectomy Past and Present Until recently, about 75 percent of all hysterectomies were performed through an incision in the abdomen. In the remaining cases, the uterus was removed.
Both procedures have drawbacks: The abdominal approach requires a 4- to 6-inch incision, results in considerable postoperative pain, requires a lengthy recuperation, and produces a visible scar. hysterectomy is not possible if the patient's ovaries must be removed, if the patient has had previous pelvic surgery, or if the surgeon must treat related disorders near the uterus.
Now, for many women facing an abdominal hysterectomy, there's a dramatic alternative: (LAVH).
The LAVH Procedure LAVH takes place under general anesthesia, so the patient is unconscious throughout the procedure. Using a trocar (a narrow tubelike instrument), the surgeon gains access to the abdomen through the navel. A laparoscope (a tiny telescope) connected to a camera is inserted through the trocar, allowing the surgeon to view a magnified image of the patient's internal organs on a video monitor. This enables the surgeon to perform the hysterectomy as well as to diagnose and treat related conditions at the same time.
Two or three additional trocars are inserted to accommodate special instrumentation. An instrument called the MULTIFIRE ENDO GIA* stapler is used to detach the uterus and seal its supporting vessels with triple rows of tiny staples. The uterus is then removed and the minute incisions are closed with sutures or surgical tape. Within a few months, the incisions are barely visible.
The Laparoscopic Revolution Laparoscopy was pioneered by gynecologists in the early 1960s, and has been widely used in a range of procedures, including tubal ligation, the removal of ovaries and fibroids (benign fibrous tumors of the uterus), and the treatment of tubal pregnancies.
In 1989, United States Surgical Corporation (USSC) developed an instrument for laparoscopic gallbladder removal that significantly reduced the pain, scarring, hospital stay, and recovery time associated with that procedure. USSC has introduced instruments for laparoscopic appendectomy; hernia repair; lung and bowel surgery; and a broad range of gynecologic procedures, including hysterectomy.
If you're concerned that laparoscopic hysterectomy is relatively new, remember that gynecologic surgeons have been using laparoscopy for the past three decades. More than 95 percent of all gallbladder surgery is now performed using this technique. Similarly, LAVH is rapidly gaining acceptance among gynecologic surgeons and their patients.
LAVH May Be for You Each year some 675,000 women in the United States undergo hysterectomy. Surgeons believe that many patients who need an abdominal hysterectomy may be treated with the laparoscopic technique. A thorough evaluation by your gynecologist can determine if LAVH is an appropriate procedure for you.
Comparison of the Outcomes of LAVH With Those of Traditional Techniques
| Laparoscopic Hysterectomy | Abdominal Hysterectomy | upper Hysterectomy | |
|---|---|---|---|
| Hospital stay | 1-2 days | 4-6 days | 3-4 days |
| Return to work | 1-2 weeks | 4-6 weeks | 3-4 weeks |
| Cosmetic results | 3-4 tiny marks | 4-6 inch scar | No visible scar |
| Recuperative pain | Minimal | Significant | Moderate |
| Ability to deal with other pelvic pathologies | Excellent | Excellent | Poor |
| Ability to remove ovaries | Easy | Easy | Difficult or impossible |
The transabdominal pre-peritoneal (TAPP) inguinal hernia repair: a trip along the learning curve
Following the laparoscopic revolution, laparoscopic hernia repair has become one of the commoner laparoscopic operations. Several studies have demonstrated a definite advantage over open repair with regard to reduced post-operative pain1-3 and earlier return to work and normal activities.1,4,5 Furthermore, when repairing recurrent hernias, it does offer an advantage of dissection in a previously non-damaged area. Bilateral hernias are repaired without extra incisions and recovery for these is very quick. However, unlike laparoscopic cholecystectomy it has not been embraced by the whole surgical community mainly because it requires increased skills and for this reason there is a longer learning curve before operating times come down to or are quicker than those for open procedures. The laparoscopic operation exposes the patient to potential complications, mainly with regard to possible visceral injury and not seen with the open approach. However, with experience, the risk of this is minimal.
Since 1992, the senior author (DSE) has performed over 1700 laparoscopic transabdominal pre-peritoneal (TAPP) inguinal repairs and recently the results have been audited by an independant observer. Over the last five years the technique has undergone many changes and refinements in response to improvements in technology and as a result of complications. We have used the audit to investigate complication rates and the effect that the various modifications have had on these. We present a description of our standard technique and also describe the complications we have experienced together with the alternations in technique which have been instituted to avoid these problems.
SURGICAL TECHNIQUE
The majority of repairs are done as a day case (75%) under general anaesthesia with overnight admission solely reserved for patients with concurrent medical problems or social difficulties, not permitting day case treatment. In an otherwise fit patient, age has been no bar to day case treatment. Previous abdominal surgery does not prevent laparoscopic repair although special precautions are taken in these patients with regard to the formation of pneumoperitoneum and trocar placement. In our experience, because of adhesions, previous left-sided colorectal surgery has led to occasional conversion to an open operation.
Following induction of anaesthesia, the supine patient is placed on the operating table with the arms to the side and in a 10-20° Trendelenburg position. This helps with the reduction of hernias and allows the intestines to gravitate into the upper abdomen. The TV monitors are placed at the patient’s feet and the Surgeon and Assistant stand on either side. A Verres needle is inserted through a small supra-umbilical incision and a pneumoperitoneum formed. A midline camera trochar (Ethicon 10mm dilating tip port) is inserted and the groins assessed. In younger patients (below 30 years) or in patients with previous abdominal surgery, an optical trochar (Visiport Auto-Suture) is used to enter the peritoneal cavity. This instrument is blunt ended with a single spring-loaded blade which cuts through tissues 1 mm at a time. With the camera inserted this allows entry into the peritoneal cavity under direct vision and avoidance of underlying or adherent structures. Following the successful placement of the camera port, a telescope is placed to trans-illuminate the abdominal wall. This allows mapping of the abdominal wall vessels and the placement of lateral ports in an avascular area. The lateral ports are usually placed in the mid-clavicular line in the region of the umbilicus, the exact placement depending upon whether the repair is to be unilateral or bilateral. The pre-peritoneal space is then entered by incising the peritoneum transversely from the region of the medial umbilical ligament laterally and anterior to the hernial defect. Peritoneal flaps are then developed. Direct sacs and small indirect sacs are fully reduced. Larger indirect sacs are part dissected and having freed the cord structures posteriorly, circumcised,. The distal part of a large sac is left insitu. The anatomy is then defined and the posterior flap fully developed, the dissection going at least 5cms posterior to the internal ring. Medially the dissection is carried to the symphysis pubis. A 15 x 10 cm mesh is then fashioned and inserted. The medial border of the mesh is adjacent to the symphysis pubis and the posterior part is placed well behind the internal ring. When the mesh is satisfactorily placed, it is stapled in place, staples being applied to the pubic bone and Cooper’s ligament. Further staples are placed into the muscle layers anteriorly but none into the ileo-pubic tract or posterior to this. If the hernia is bilateral, the same procedure is performed on the contra-lateral side, a second mesh being used. The peritoneum is then reconstituted by stapling and the operation completed by closing the external oblique at the port sites and placing subcutaneous sutures to the skin.
FOLLOW-UP
Patients are discharged either on the day of operation or on the following day, others occasionally having to remain in hospital because of previous medical conditions. Patients are instructed that if they are pain free they can drive a car any time from two days post-operatively. They can return to sedentary jobs after four days and to full manual jobs after ten days. All repairs are reviewed in the clinic two weeks post-operatively and any early complications noted. The initial 700 repairs were subsequently audited by an independent observer in 1996 by direct interview and examination (67%) or by questionnaire (33%) to non attendees. In this way we were able to document accurately all complications year by year and the effects that subsequent modifications of the technique had.
Bleeding
Minor bruising around the port sites is common and does not appear to cause any problems to the patient. However, early on we experienced several episodes of major abdominal wall bruising which required the patient to remain in hospital or to be readmitted following discharge. This appeared to be related to damage to subcutaneous vessels or branches of the inferior epigastric artery during lateral port insertion. After 140 patients, we began to transilluminate the abdominal wall with the telescope light prior to port insertion. This allows visualisation of the abdominal wall vessels and insertion of the trochars into avascular areas. Since then there has been only one further case of massive abdominal wall bruising in over 1500 repairs. Scrotal bruising was initially a significant problem and occurred in patients with large inguino-scrotal hernias. Initially, these large indirect sacs were dissected completely off the cord structures, often causing delayed venous bleeding. Since the technique was changed and sacs were circumcised in relation to the internal ring with the distal sac left in situ, this complication has diminished. However, the distal sac can fill with fluid giving a seroma and often causing the patient to return to the GP complaining of a scrotal swelling which the GP on occasionshas diagnosed as a recurrence. Examination demonstrates a well defined seroma in the sac remnants. About 11% of patients have had this complication but the majority settle and absorb spontaneously within 2-3 months. Only a very few have required eventual aspiration. This complication is commonly reported and patients are now warned that there is a residual sac in situ and that this is a possibility.
Visceral Injuries
One patient early in the series, after a number of previous abdominal operations and despite being warned of the increased risks, requested a laparoscopic repair. A hole was made in the small bowel which necessitated conversion. Now, in patients who have previously undergone abdominal surgery, an optical trochar (Visiport Auto-Suture) is used for initial entry into the abdomen.
Small Bowel Obstruction
We have had three cases of small bowel obstruction following laparoscopic hernia repair, two patients in the first 450 and one since. In the first case, diagnosed at laparotomy, a defect had appeared in the peritoneum overlying the mesh, allowing a loop of small bowel to become incarcerated. The second and third cases in which there was an internal hernia were treated laparoscopically, the patients returning home 48 hours after the second procedure. To minimise the risk of this happening, we ensure that extensive peritoneal flaps are created so that the peritoneum can be reconstituted at the end of the procedure by staples or sutures and that they are free of tension.
Port Site Hernias
In 1992, when reusable ports with pyramidal trochars were used, 7.7% of patients developed port site hernias. After changing to disposable ports, again with pyramidal trochars, the incidence dropped to 3.2%. However, since early in 1997, we have used dilating tip ports with a linear blade the incidence of this complication has diminished and we have yet to see a further hernia.
Urinary Retention
As in open hernia repair this is not an uncommon complication and one of the commonest reasons for delayed discharge in day case patients. However, with recognition of the problem we now mobilise patients early after operation and encourage urination. This has resulted in an admission rate of 1% because of retention for our day case patients.
Recurrence
Recurrence of hernias has been the benchmark by which all repairs are measured. In the first 100 hundred patients, we experienced an unacceptable 9% recurrence rate. At this time a 6 x 5 cm mesh was inserted over the site of the hernia and stapled to the surrounding tissues. It was not fixed to the symphysis pubis or inguinal ligament. In 1993, the mesh size was increased to 12 x 7 cms and this immediately reduced the recurrence rate to 2.9% in patients operated on or before the end of 1995. Since changing to 15 x10 cm mesh in early 1996 no recurrences have been recorded although the follow up period is obviously shorter. In order to accommodate the size of mesh, a large pre-peritoneal pocket is created behind the inguinal canal as far medially as the symphysis pubis and laterally several centimetres beyond the deep ring. Posteriorly, this is carried 5 cms behind the internal ring. This large pocket allows the mesh to sit flat against the overlying tissues and to be stapled to the symphysis, Coopers ligament, the lateral edge of the linea and superiorly along the back of the arcuate ligament but not stapling the inguinal ligament or posterior to it.
Meralgia Paraesthetica
Early in the series, a small number of patients complained of lateral thigh pain compatible with meralgia paraesthetica due to compression of the lateral cutaneous nerve. The majority settled spontaneously but one required operation. The incidence of this condition has now disappeared since we stopped stapling the ileo-pubic tract or posterior to it. This complication has been recognised in the literature from an early stage and guidance given on its avoidance.6
ADVANTAGES OF LAPAROSCOPIC HERNIA REPAIR
Speed of Repair
Unlike many previous reports, in our audit the time to do a laparoscopic repair from skin incision to final stitch is as quick, or quicker than an open repair. In several hundred cases the average time for a unilateral repair has been 25 minutes and 38 minutes for a bilateral repair. These times have allowed increased through put through the Day Surgical Unit.
Indentification of Bilateral Herniae
Over 200 simultaneous bilateral repairs have been done and the operation has been well tolerated with no increase in morbidity and low levels of post-operative pain. In patients admitted to hospital for a unilateral repair, more than 20% have been found on laparoscopy to have a significant hernia on the side found to be normal on clinical examination. Many of these have been repaired but when not, patients have returned subsequently with a symptomatic contra-lateral hernia requesting repair. We are now undertaking a prospective randomised trial to determine whether these unsuspected defects should also be repaired at the same operation as this may be beneficial to both the patients and the hospital waiting list.
Post-operative Pain
The perceived advantages of the laparoscopic approach to hernia over the open version is a reduction in post-operative pain and an early return to normal activities. Several studies using pain scores have validated this advantage with low levels of pain.3,5,7 We did not use a pain score in our audit but did record perceived pain in post-operative interviews. Sixty percent of patients stated that they had suffered little or no pain and 40% used no analgesia at all when discharged. Return to normal activity was rapid; the average time to driving was 12 days with some patients driving within 2 days and one flying a light aircraft in 5 days. Patients have returned to sedentary jobs early and to heavy manual work, on average, 18 days after operation. The major factor in determining return to work has been the GP and, on questioning, we have found that many patients felt able to return to work before the GP permitted it. We have increased GP education in this matter and this has reduced the length of convalescence for these patients further.
WHEN TO GO OPEN
If following induction of anaesthesia the hernia is still irreducible, despite gentle retraction with atraumatic Babcocks and external pressure, we recommend conversion to an open operation. In the past, when we have persisted with dissection in these patients, it has usually resulted in significant bruising and a difficult repair. A trial dissection is indicated in elective cases but if the hernia is irreducible in the emergency case then the operation should be open. Similarly, an open procedure should be considered in patients with previous complicated pelvic surgery, for instance a Hartmann’s procedure, as a laparoscopic approach is likely to be hazardous. Patients who have significant cardiovascular disease or severechronic obstructive pulmonary disease should also have an open procedure under local or regional anaesthesia as general anaesthesia and a pneumoperitoneum carry a significant risk in these conditions.
DISCUSSION
Laparoscopic hernia repair was first described by Ger in 1990, who placed a simple mesh plug in the defect.8 The technique has undergone a significant metamorphosis during the last few years. Currently, there are two types of laparoscopic hernia repair; the transabdominal pre-peritoneal (TAPP) repair (as described in this article) and the totally extraperitoneal (TEP) repair. The TEP involves creation of an extraperitoneal space posterior to the inguinal canal either with a Verres needle or more commonly a balloon and place-ment of a mesh in a similar fashion to the TAPP repair. The TEP may have some advantages over the TAPP in terms of postoperative pain and reduced potential for intraperitoneal complications but does require a high level of technical skill associated with a considerable learning curve.9,10 We prefer TAPP repairs as they are technically easier, provide a better view of the anatomy and do not require further equipment beyond that normally available in most departments performing laparoscopic cholecystectomy. Several studies have demonstrated a clear advantage of laparoscopic hernia repair over open repair in terms of reduced post-operative pain and earlier return to work and normal activities.2,3,5,7,11 Despite this, the laparoscopic approach has been slow to gain popularity amongst many surgeons. This, we believe is due to a number of factors. Firstly, more advanced technical skills are required compared with a Lichenstein repair. Secondly, the long-term results of the laparoscopic repair are unknown. Thirdly, there is the potential for complications not seen with an open repair and, finally, the operation itself is very new and has undergone considerable refinement in both technique and equipment used. Nowadays, the majority of general surgeons do have quite extensive experience with laparoscopy in cholecystectomy and this is sufficient to be able to perform a TAPP repair. Recurrence rates are very low with both the open and laparoscopic mesh repairs with randomised studies showing no difference between the two.5,12 In these studies, as in ours, large meshes are used and this is an important factor in reducing recurrence. However, 10 and 15 year recurrence rates are obviously not available with the laparoscopic approach at this time. Complications with experience and technical improvements are now minimal in the laparoscopic repair and studies indicate similar complication rates between open and laparo-scopic repairs.2,7 Open repairs appear to have a higher rate of groin haematoma and genital oedema. One disadvantage of the laparoscopic repair is an increase in cost because of the equipment required1, but with earlier return to work we believe this cost is outweighed by the benefits to patient and society. A major advantage of the laparoscopic appproach is the ability to detect and repair a contralateral defect at the same operation with only a moderate increase in operating time.
As with laparoscopic cholecystectomy there is a definite learning curve, but we anticipate that with general laparo-scopic experience the learning curve for hernia repair will become short. We would hope that by adopting measures outlined in this paper surgeons wishing to perform laparoscopic hernia repair will join the learning curve further on, thus minimising the chances of complications seen during the development of this procedure.
CONCLUSION
Since 1992 the TAPP hernia repair has significantly evolved into a safe, effective operation. The main technical considerations that we have instituted have been the use of a large retroperitoneal pocket and large mesh, closure of the fascia at port sites, transillumination of the abdominal wall to avoid vascular injury and the liberal use of the optical trochar in patients with previous surgery and adhesions as we feel this may help to reduce the risk of visceral injury during trochar insertion.





