Thesis Submitted by DR. TUAN CIN THANG (M.MAS-CANDIDATE)
APRIL 2025
ABSTRACT:
Introduction: Laparoscopic cholecystectomy has become gold standard because of its well-recognized minimal invasiveness and accelerated post-operative recovery. The most common use of energy sources in laparoscopic cholecystectomy are conventional electrosurgery and harmonic scalpel. However, the conventional electrosurgery produces excessive smoke and the intense collateral heat generation leading to tissue necrosis and subsequent perforation of gallbladder resulting in bile and stone spillage which will interfere with the laparoscopic view and leads to frequent instrument changes, and ultimately prolongs operating time. An advanced energy source, harmonic scalpel may provide the advantage of shorter operating time by reducing smoke and frequency of changing working instruments and provide bloodless dissection in GB bed. This study assesses whether the use of the expensive harmonic scalpel in laparoscopic cholecystectomy is justifiable.
Aim: To analyze the surgical outcomes of harmonic scalpel assisted versus conventional laparoscopic cholecystectomy.
Methods: This is a retrospective cohort study compared harmonic scalpel assisted laparoscopic cholecystectomy (HLC) and conventional laparoscopic cholecystectomy (CLC) in terms of surgical outcomes. Data of patients who underwent LC using either harmonic scalpel or monopolar diathermy were collected and categorized into two groups. In group A, harmonic scalpel was used for dissection and monopolar electrosurgery was used in group B. Outcome parameters analyzed were mean operation time, blood loss, bile duct injury, drain placement, conversion to open surgery, post-operative pain using visual analogue scale (VAS) scoring, nausea and vomiting, surgical site infection, need of ERCP, re-operation, length of hospital stay, mortality and cost per procedure (USD).
Results: The mean operative time was significantly shorter in the HLC group than the CLC group (38.2 5.5 min vs 46.8 6.2 min, p<0.0001). The mean intraoperative blood loss was also significantly lower in the HLC group (17.9 6.0ml vs36.4 7.9ml, p<0.00001). Furthermore, the HLC group showed significantly lower postoperative pain scores at all time points (p<0.00001). The duration of hospital stay was significantly shorter in HLC (1.4 0.6 days vs 2.3 0.7 days, p<0.0001). Nevertheless, the expense of cost per procedure was significantly higher in the HLC group ($300 50 vs $100 20, p<0.00001). Other parameters such as drain placement (15% vs 35%, p=0.273), bile duct injury (0% vs 10%, p=0.487), conversion to open surgery (0% vs 10%, p=0.487), postoperative nausea and vomiting (15%vs30%, p=0.451), need for ERCP (5% vs15%, p=0.605), re-operation (0%vs5%, p=1.000), SSI (10%vs20%, p=0.661) did not significantly differ between the groups.
Conclusion: In this study, apart from its high cost, HLC has significant advantages over CLC with respect to operating time, intraoperative blood loss, postoperative pain and length of hospital stay.
CHAPTER 1
INTRODUCTION
The first person to perform laparoscopic cholecystectomy was Professor Muhe of Boblingen, Germany. He performed the operation on September 12, 1985.1 It was popularized in the late 1980s by Mouret and Dubois in Europe and Reddick in the United States.2. Since then, laparoscopic cholecystectomy has undergone various developments. This includes the use of multiple techniques for coagulation during dissection. Now, laparoscopic cholecystectomy has replaced conventional open cholecystectomy and has become the gold standard.1 The reason may be its well-recognized minimal invasiveness and accelerated post-operative recovery. 3 Indications for cholecystectomy include cholecystitis (acute and chronic), biliary dyskinesia, adenomatous gallbladder polyps, gall stone diseases.4 The coagulation during dissection can be achieved by conventional electrocoagulation or the harmonic scalpel, which uses ultrasonic energy.1
CHAPTER 2
OBJECTIVES
General Objective
CHAPTER 3
LITERATURE REVIEW
3.1. Acute Cholecystitis (AC)
It is inflammation of gallbladder results from obstruction of the cystic duct, usually by a gallstone, followed by distension and subsequent chemical or bacterial inflammation of the gallbladder. People with acute cholecystitis usually have persistent right upper quadrant pain, anorexia, nausea, vomiting, and fever. About 95% of people with acute cholecystitis have gallstones (calculous cholecystitis) and 5% lack gallstones (acalculous cholecystitis). Severe acute cholecystitis may lead to necrosis of the gallbladder wall, known as gangrenous cholecystitis11.
The incidence of acute cholecystitis among people with gallstones is unknown. The incidence of acute cholecystitis is about 20% among people with biliary colic. Biliary colic occurs in 1% to 4% of people with gallstones12. About 20% of patients admitted to hospital for biliary tract disease have acute cholecystitis. Acute calculous cholecystitis is three times more common in women than in men up to the age of 50 years, and is about one and a half times more common in women than in men thereafter.11
Acute calculous cholecystitis seems to be caused by obstruction of the cystic duct by a gallstone, or local mucosal erosion and inflammation caused by a stone, but cystic duct ligation alone does not produce acute cholecystitis in animal studies. The role of bacteria in the pathogenesis of acute cholecystitis is not clear; positive cultures of bile or gallbladder wall are found in 50% to 75% of cases.13,14The cause of acute acalculous cholecystitis is uncertain and may be multifactorial, including increased susceptibility to bacterial colonization of static gallbladder bile.11
3.2. Treatment
The treatment of AC is based on the disease severity, the presence of complications, and pre-existing conditions and comorbidities. Early LC represents the cornerstone in the treatment of AC, but, in some circumstances, when early LC is contraindicated, delayed surgery is performed. Medical treatment, especially the use of antibiotics, plays a crucial role. Sometimes, gallbladder drainage (GBD) may be indicated15. In difficult conditions, subtotal cholecystectomy may be necessary due to not reaching the critical view of safety, difficulty identification of the anatomical structures with risk of injury to nearby structures 16.
3.2.1. Medical Treatment
In the course of AC, patients should be kept on fasting and initiate antimicrobial therapy. General supportive care, such as fluid and electrolyte intravenous infusion, and possibly analgesic agent administration, are also mandatory 17.
In order to select a suitable empirical treatment, generally based on broad-spectrum antibiotics (e.g., penicillin, cephalosporins, fluoroquinolones), clinicians should consider drug pharmacokinetics and pharmacodynamics, local antibiogram, a history of antimicrobial use, allergic or adverse reactions, and renal and hepatic function. Importantly, the presence of a biliary–enteric anastomosis warrants anaerobic therapy (e.g., metronidazole) 18. A culture of bile and gallbladder tissue is suggested during cholecystectomy in case of emphysematous cholecystitis, gallbladder wall necrosis, or perforation 19. Once cultures and susceptibility test results are available, clinicians should discontinue antimicrobial therapy if no longer needed or switch to an antimicrobial agent that is specific for the isolated organism. The duration of antibiotic therapy depends on clinical features18.
3.2.2. Surgical Treatment
(a) Laparoscopic Cholecystectomy
The cornerstone of AC treatment is early LC. In particular, early LC performed within 72 hours should be the method of choice for the treatment of AC, because it is related to a shorter hospital stay, fewer perioperative complications, and reduced costs18.
(b) Gallbladder Drainage
GBD, also known as cholecystostomy, should be performed in all patients with severe AC in whom cholecystectomy is contraindicated. Moreover, GBD should also be considered in patients with moderate AC and a high surgical risk, particularly in case of an inadequate response to the medical treatment 15. Percutaneous transhepatic GBD, performed under ultrasound guidance, is the method of choice. In contrast, percutaneous GBD through the transperitoneal route is not recommended, because it is associated with a higher rate of complications, mainly bile leakage and biliary peritonitis20
(c) Subtotal Cholecystectomy
Subtotal cholecystectomy is a bail-out procedure undertaken when facing difficult laparoscopic cholecystectomy due to not reaching the critical view of safety, inadequate identification of the anatomical structures involved and/or risk of injury 16.
The 2020 World Journal of Emergency Surgery guide for acute calculous cholecystitis recommends performing subtotal cholecystectomy in situations where it’s difficult to identify the anatomical structures needed or if there is a high risk of iatrogenic lesions21.
3.3. Energy Sources Used in Laparoscopic Cholecystectomy
In LC, the common energy sources used are electrosurgery and harmonic scalpel.
3.3.1. Electrosurgery
In electrosurgery, heat is generated in the tissue by the flow of radio frequency (RF) electric current. The RF energy can be applied to tissue by using either monopolar or bipolar tools. In monopolar electrosurgery, the electrical circuit is completed by the passage of current from the active electrode at the surgical site to the dispersive electrode (or the return electrode) attached to the body of the patient. The active electrode can be of any form (usually a point, hook or a blade) with sharp edges and/or blunt edges. The return electrode is usually a wide pad, attached to the skin of the patient, which disperses the heat and safely leads the current out of the body. The waveforms with different duty cycles can be used to produce four main effects inelectrosurgery namely, cutting, coagulation, desiccation and fulguration. To perform coagulation or desiccation, a lower duty cycle high voltage waveform is used but can also be performed with 100 % duty cycle lower voltage cutting waveform as well. Finally in fulguration, a lower duty cycle high voltage waveform is applied through the active electrode of a pointed monopolar electrosurgical tool tip in noncontact mode close to the tissues.
The current travelling through the body can interfere with any implanted medical devices such as pacemakers and defibrillators. Other mechanisms through which injuries can occur during electrosurgery include insulation failure, remote injury, direct and capacitive coupling.
3.3.1. Ultrasonic Energy
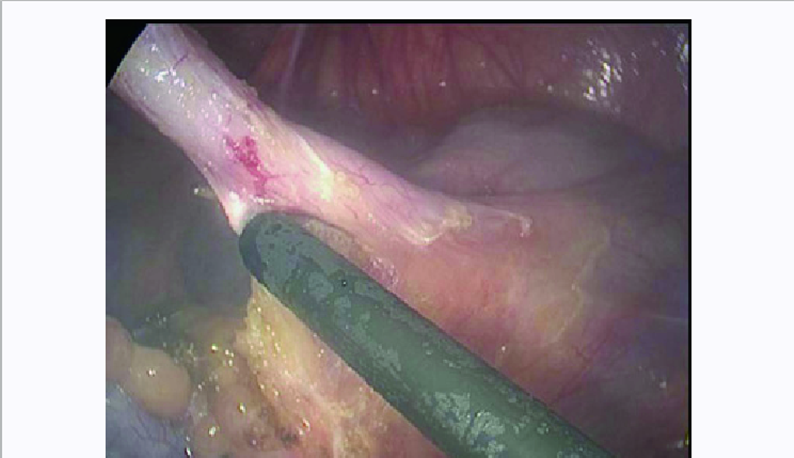
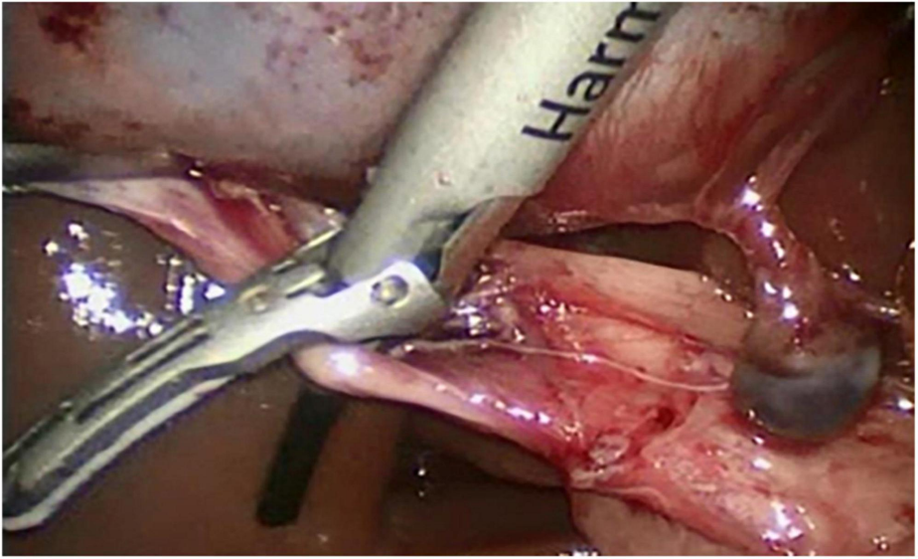
The basic working principle of ultrasonic surgical instruments such as ultrasonically activated scalpel (UAS) is to use the low frequency mechanical vibrations (ultrasonic energy in the range of 15–55 kHz) of the tool tips or the blades for tissue cutting and coagulation. The mechanical vibrations when transferred to the tissues on contact induces protein denaturation by breaking down the hydrogen bonds in tissues due to the internal cellular friction caused by the vibrations. The mechanical vibrations are produced by the piezoelectric transducers embedded in the tools which convert the applied electrical energy to mechanical vibrations which are then transferred to the active blades for cutting or coagulation. In general, the cutting and coagulation in UAS depends on various factors such as grip pressure, the shape and area of the blades in contact with the tissues and the power settings.
The major advantage of using UAS is that it produces less heat compared to other energy devices (temperature by harmonic scalpel, 80-100 compared to 200-300 for electrocoagulation) thereby reducing the risk of thermal injury. Due to lesser heat generation, charring and desiccation is also greatly reduced. Since no smoke is produced, except for the mist produced due to cavitation effect which dissipates much faster, UAS offers unobstructed view for endoscopic/ laparoscopic procedures. The UAS does not transmit active current in the tissues and thereby eliminate any risk of electric shock.
Not many complications were reported in the use of harmonic scalpel in laparoscopy. General disadvantages of ultrasonic devices include slower coagulation compared to electrosurgery, altering of the frequency or impedance of the surgical system itself due to blade fatigue, temperature elevation, excessive applied pressure, or improper use22,23. Moreover, if the vibrating jaw directly touch bowel or blood vessels, it can be punctured23.
3.4.1. Surgical Management of Cholecystitis
It can be done by open or laparoscopic approach. Nevertheless, Open cholecystectomy (OC) is being phased out in favor of laparoscopic cholecystectomy (LC) as a treatment for AC. From 0% in 1987 to 80% in 1992, the proportion of cholecystectomies done laparoscopically has increased. Due to the progress of laparoscopic technology, the growing competence and experience of surgeons, shorter hospital stays, and a shorter period for return to regular activities, open operations have been replaced by laparoscopic methods24.
3.4.1. Laparoscopic Cholecystectomy
(a) Patient position
The patient is operated in the supine position with a steep head up and left tilt. This typical positioning of laparoscopic cholecystectomy should be achieved once the pneumoperitoneum has been established. The patient is then placed in reverse Trendelenburg’s position and rotated to the left to give maximal exposure to the right upper quadrant.

Figure 3: Patient Position in Laparoscopic Cholecystectomy
(b) Position of Surgical Team
The surgeon stands on the left side of the patient with camera holder-assistant. A scrub nurse and one assistant stand right to the patient and should hold the fundus grasping forceps.
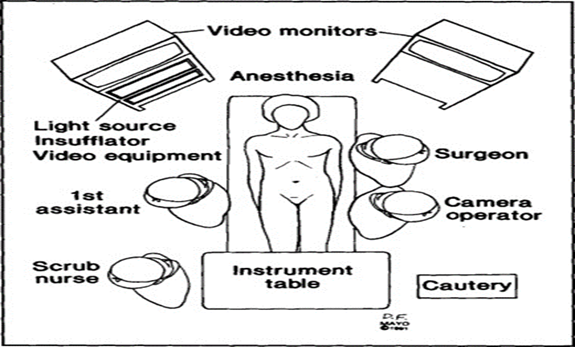
Figure 4: Position of Surgical Team
(c) Steps of Procedure
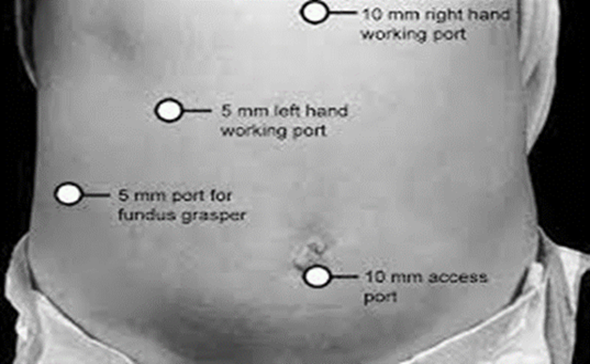
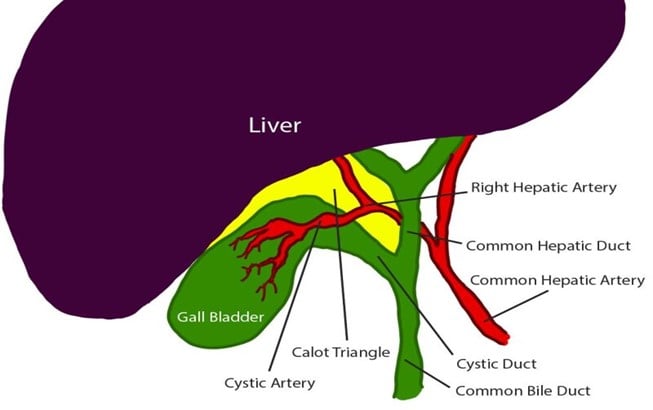

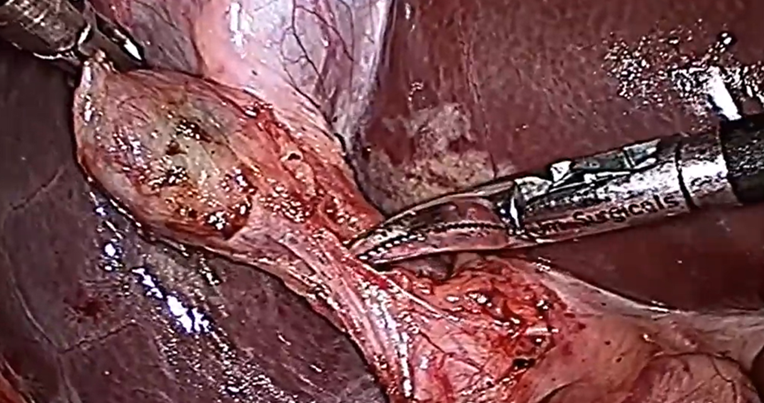
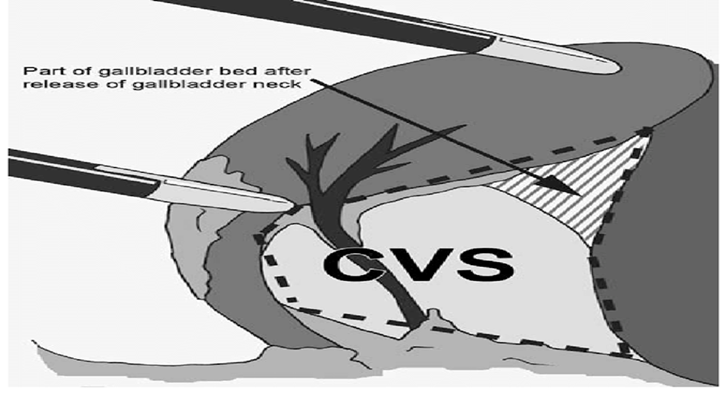
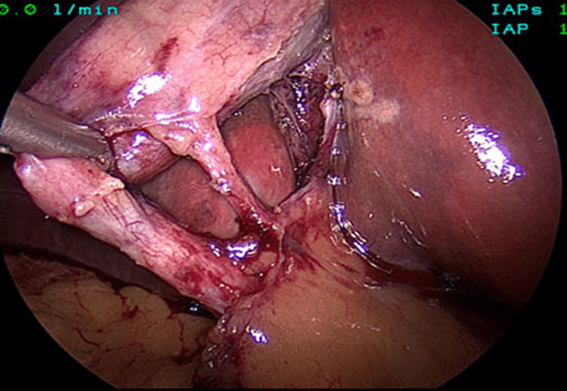
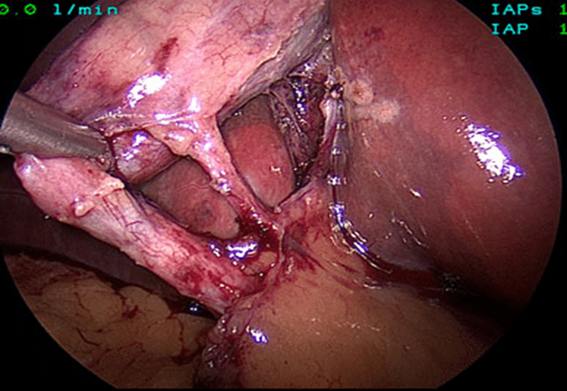
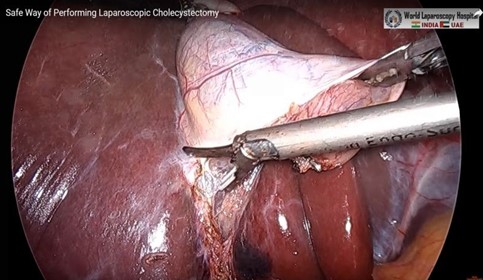
The mean duration of surgery in the CLC group was 55.5±14.1 minutes, and in the HLC group was 46.8±14.3 minutes. The difference in the duration of surgery between the two groups was significant with p-value of 0.0249. Bessa et al. conducted a prospective study of 120 patients with gall bladder disease undergoing laparoscopic cholecystectomy. These patients were assigned either to the harmonic scalpel group (HS group = 60) or the clip and cautery group (C&C=60).The median operation time was statistically significantly shorter in the HS group than in the C&C group (32 vs. 40 minutes, respectively; P = 0.000)1. In 2014, Catena et al. had done a randomized controlled trial. A total of 42 patients were assigned randomly to either the harmonic scalpel group (H = 21) or the monopolar diathermy group (MD = 21). The intraoperative blood loss was significantly less in the harmonic scalpel group (91.1 ± 11.9 vs 166.6 cc ± 19.2, p<0.05). The conversion rate was also significantly less in the harmonic scalpel group(4.7% vs 33%, p<0.05)10. In the study of A.Zanghi et al., 121 patients in whom dissection and coagulation were performed using monopolar diathermy and 43 patients who were all treated with the harmonic scalpel ( Ethicon Endo-Surgery) as the sole instrument used in the whole procedure. The intraoperative volume blood loss was significantly more in the traditional group than in the harmonic scalpel group (29.32+14.21 vs. 12.41+8.22; p < 0.0001)4. In the study of Liao et al., a total of 198 patients were randomly allocated to LC with a Harmonic scalpel (experimental group, 117 patients) or conventional monopolar electrocautery (control group, 81 patients). The conversion to open cholecystectomy was required in 1 patient of the experimental group caused by common bile duct injury, whereas none of the patients from the control group underwent conversion (P >0.05)3. In the study of Rajnish et al, out of 40 patients, an intraperitoneal drain was kept in seven patients (17.5%). Six patients (30%) in the CLC group and four patients (20%) in the HLC group required an intraperitoneal drain. The difference was not significant (p = 0.716).
This is a hospital based retrospective cohort study.
All patients with gallbladder diseases underwent laparoscopic cholecystectomy by using either monopolar diathermy or harmonic scalpel in World Laparoscopic Hospital in the study period. Patients were selected based on the following inclusion and exclusion criteria.
- Age between 20 and 70 years
- Physical status ASA class I or II
- Diagnosis of simple acute or chronic cholecystitis
- Cholelithiasis
- Gallbladder polyps
- Suitability for LC because of the presence of clinically significant symptoms
- Prophylaxis of malignancy in high-risk patients
- BMI <40 kg/m2
- Age <20 years and >70years
- Pregnant or lactating women
- BMI >40 kg/m2
- ASA class III or IV
- Complicated intrahepatic or extrahepatic bile duct stones
- Complicated acute pancreatitis
- Suspected gallbladder malignancy
- History of previous open upper abdominal surgery
Preoperative, intraoperative and postoperative data from medical records, who underwent laparoscopic cholecystectomy using either harmonic scalpel or conventional monopolar diathermy in World Laparoscopic Hospital, were collected according to inclusion and exclusion criteria consecutively during the study period.
Sample size calculated from the following formula.
- Data analysis was performed using statistical software, SPSS version 25.
- Mean and Standard Deviation for continuous variables
- Frequencies and percentages for categorical variables
- Independent t-test for continuous variables
- Fisher’s Exact test for categorical variables
Preoperative, intraoperative and postoperative data from medical records, who underwent laparoscopic cholecystectomy using either harmonic scalpel or conventional monopolar diathermy in World Laparoscopic Hospital, were collected according to inclusion and exclusion criteria consecutively during the study period. The sample was calculated as 20 in each group.
The demographic profiles in both groups were noted. The intraoperative parameters such as duration of surgery (skin to skin), amount of blood loss, presence or absence of injury to bile duct, drain placement and conversion to open surgery were noted in both groups. Postoperative pain was assessed using the visual analogue scale (VAS) score at 1,2,4,6 and 24 hours. Moreover, other postoperative parameters such as postoperative nausea, vomiting and need of ERCP, reoperation, duration of hospital stay, cost per procedure and mortality were recorded. At the time of discharge, during the first postoperative visit and at the 30th postoperative day, patients were examined for wound infection. After collecting the patient data, they were analyzed to compare the outcomes between the two groups.
A total of 40 patients were enrolled in the study, 20 in harmonic scalpel LC and 20 in conventional LC. The demographic profiles of the two groups were comparable. The mean age for the group undergoing HLC (group A) was 43.3±11.22 years, ranging from 25 years to 62 years. The mean age for the CLC group (group B) was 44.75±10.85 years, ranging from 26 years to 64 years. This data suggests that gallstone is most common in the age group of 40-50 years. Out of the total 40 patients enrolled in the study, 29 (72.5%) were females and 11(27.5%) were males and the gender ratio in both groups was comparable. Both groups had a comparable body mass index (BMI) and distribution of various comorbidities in the study population. The American Society of Anesthesiologist (ASA) fitness category did not significantly differ between the two groups (Table-1).
| Parameters | Group A(n=20) | Group B(n=20) | |
| Age (mean SD) | 43.3 11.22 | 44.75 10.85 | |
| BMI (mean SD) | 26.43 4.75 | 26.62 4.22 | |
| Sex | Male Female |
6(30%) 14(70%) |
5(25%) 15(75%) |
| Comorbidities | Diabetes Hypertension |
8(40%) 5(25%) |
5(25%) 15(75%) |
| ASA Category | ASA I ASA II |
11(55%) 9(45%) |
12(60%) 8(40%) |
| Group A(HLC) | Group B (CLC) | p-value | |
| Mean operation time | 38.2±5.5 min | 46.8±6.2 min | <0.0001 |
| Mean blood loss | 17.9±6.0ml | 36.4±7.9ml | <0.00001 |
| Mean blood loss | 3(15%) | 7(35%) | 0.273 |
| Bile duct injury | 0(0%) | 2(10%) | 0.487 |
| Conversion to open surgery | 0(0%) | 2(10%) | 0.487 |
Table 3: Comparison of Postoperative Pain Using VAS Score Between the Groups
| Pain Score (VAS 0-10) | HLC | CLC | p-value |
| 1 Hour | 4.1±0.8 | 5.3±1.0 | <0.001 |
| 2 Hours | 3.5±0.7 | 4.8±1.1 | <0.00001 |
| 4 Hours | 3.0±0.6 | 4.4±1.0 | <0.00001 |
| 6 Hours | 2.6±0.5 | 3.9±0.9 | <0.00001 |
| 24 Hours | 2.2±0.5 | 3.5±0.8 | <0.00001 |
| HLC Group | CLC Group | p-value | |
| Nausea and Vomiting | 3(15%) | 6(30%) | 0.451 |
| Surgical Site Infection | 2(10%) | 4(20%) | 0.661 |
| Need for ERCP | 1(5%) | 3(15%) | 0.605 |
| Re-operation | 0 (0%) | 1(5%) | 1.000 |
| Length of Hospital Stay | 1.4±0.6 days | 2.3±0.7 days | <0.0001 |
| Mortality | 0 (0%) | 0(0%) | |
| Cost Per Procedure(USD) | $300±50 | $100±20 | <0.00001 |
In Sasi et.al. study , postoperative nausea was significantly lower with ultrasonic dissection compared with electrocautery(p<0.05)25. However, Kandil et al study showed that the incidence of nausea and vomiting was higher in the diathermy group. But it did not show a significant difference (p>0.05). In the present study, occurrence of postoperative nausea and vomiting were not statistically different (p=0.451). In the Tsimoyiannis et al study, three patients needed ERCP for postoperative bile leakage from the gallbladder’s liver bed in the electrocautery group. But there is no statistical difference between the two groups with regards to this outcome (P=0.19). In the current study, three patients in CLC group needed ERCP for stent placement due to occurrence of symptomatic biloma presented after two weeks of operation. In HLC group, one patient required ERCP stent placement for symptomatic biloma presented after 10 days of surgery. After stent placement, all four patients were recovered well. Cholangiogram was taken after four weeks and there was no more bile leakage in all patients. So, stents removal was done in all patients. However, the need of ERCP in the two groups was not statistically significant (5%vs 15%, p=0.605). Meanwhile, one patient with CBD injury from the CLC group had progressive bile leakage with signs and symptoms of biliary peritonitis in the postoperative period and reoperation was done on fifth postoperative day. Roux-en-Y hepaticojejunostomy was performed and postoperative period was uneventful. The risk of SSI is less in laparoscopic procedures as compared to open surgeries, as the size of the incision is small. The risk of SSI depends on various factors, such as duration of surgery, spillage of bile, intra-abdominal collection due to postoperative bile leak, retrieval of GB through the port, presence of drain, and comorbidities such as diabetes6. In the study of Rajnish et al, three patients (15%) in the CLC group had superficial SSI and two patients (10%) in the HLC group had superficial SSI. The SSI rates between the two groups were not significant (p = 1.000). In Sasi et al. study, port-site infections occurred in two patients in the ultrasonic group (3.3%) and in 3 others in the electrocautery group (5%). This outcome was not statistically significant(p>0.05). In this study, two patients (10%) in the HLC group had superficial SSI and both of them were diabetics. Four patients (20%) in CLC group developed superficial SSI. Among them, three patients had diabetes and one patient had an intraperitoneal drain. The overall SSI was 15% and test was not significant (10%vs20%, p=0.661). There was no mortality in the study groups.
The primary objective of this study was to analyze surgical outcomes of harmonic scalpel laparoscopic cholecystectomy versus conventional monopolar diathermy laparoscopic cholecystectomy. The HLC group demonstrated significantly lower operative time, intraoperative blood loss, length of hospital stay, and postoperative pain scores compared to the CLC group. However, there were no significant differences between the two groups in terms of bile duct injury, conversion to open surgery and the need for drain placement. Postoperative outcomes such as nausea and vomiting, the need of ERCP intervention, surgical site infection, and reoperation rates were also comparable. Notably, HLC offered consistently lower pain scores across assessed time points. Despite these advantages, the primary limitation of HLC remains its higher cost, which poses a significant challenge, particularly in resource-limited settings and developing countries.