Comparative study to evaluate the role of ICG vs lighted ureteral Stent
COMPARATIVE STUDY TO EVALUATE THE ROLE OF ICG DYE VS LIGHTED URETERAL STENT (U KIT) TO PREVENT INTRAOPERATIVE URETERIC INJURY UNDER NIR FLUORESCENCE IN TOTAL LAPAROSCOPIC HYSTERECTOMY" MASTER IN MINIMAL ACCESS SURGERY
World Laparoscopy Hospital
DECEMBER 2020 to NOVEMBER 2021
ACKNOWLEDGEMENT
I express a deep sense of gratitude and regard to my esteemed and honorable teacher Dr. R.K. MISHRA for his constant guidance and suggestions, which have made this dissertation possible. I am also thankful to Dr. VIVEK BINDAL, Dr. NITEEN GHORPADE, Dr. SWAPNIL MANE, Dr. PRAKASH AGHAV for their support in this study. I am also thankful to my parents, Dr. Anjana Kumari, Dr. Amarendra Kumar, my aunt, Archana Roy, and to all the paramedical staff and technicians of WLH, INSPIRIA HOSPITAL RAHATA, SAIDHAM HOSPITAL for their help in various ways for this study. I thank all my patients and their relatives whose cooperation during this study was invaluable and without which this study would not have been possible. Dr. ANVESHA KUMAR M.S. (Obs. & Gyane).World Laparoscopy Hospital
DECEMBER 2020 to NOVEMBER 2021
ACKNOWLEDGEMENT
INTRODUCTION:
Laparoscopic surgery has become widely accepted by surgeons and patients as an effective technique to treat gynecologic pathologies.1 Better recovery, a shorter hospital stay, less postoperative pain, and lower blood loss are the main arguments in favor of this approach.2-4 As the technology has improved and surgical skills have increased, the nature and characteristics of laparoscopic procedures have also become more complex. At centers equipped for advanced laparoscopic surgery, procedures such as surgery for complex adnexal lesions, hysterectomies, pelvic floor repair, and resection for severe endometriosis are now performed by this approach.5 Today more and more classic gynecologic operations are being replaced by laparoscopy, like tubal ligation, adnexal surgery, myomectomy, hysterectomy, and even cases of gynecologic cancer. Despite the numerous advantages it provides, it should not be considered a panacea, as it remains a surgical procedure and has the risk of complications as every other procedure. As the complexity of gynecologic cases approached laparoscopically continues to increase, it is imperative that surgeons be aware of the risk of ureteral injury. Although the overall incidence of ureteral injury is low in 2 gynecologic laparoscopies, the potential morbidity for the patient merits vigilance. Iatrogenic ureteral injury (IUI) is a serious surgical complication, with a reported overall incidence of up to 1.2%.6 The highest prevalence of IUI is recorded in gynecological procedures (50% of IUIs), followed by urologic (30%), and colorectal surgery (5–15%).7
Unfortunately, most IUIs are identified post-operatively.8 If they were detected intraoperatively, this would allow for immediate repair and improved outcomes.9 Intraoperative ureter identification is most often achieved by visual l inspection and palpation. Both can be more challenging during laparoscopic procedures, translating into a higher risk of IUI.10 Currently, the success of NIRF imaging is based upon the ability of the device to detect ICG in humans with the expectation that “first-in-human” molecularly targeted imaging agents can likewise be detected by these devices already employed to detect ICG. ICG is comparatively dim compared with other emerging NIR fluorophores which promise brighter fluorescent signals and the ability to detect targeted tissues deeper and with smaller concentrations than is used for haemo- and lymphovascular imaging of ICG. Device performance is critical and depends upon optimal excitation of NIRF imaging agents, rejection of backscattered excitation and ambient light, and selective collection of fluorescence emanating from the fluorophore.
A particularly critical issue in minimally invasive surgery is that unnecessary tissue manipulation or accidental trauma may cause ischemia and may compromise ureteropelvic or ureteral reparation. There is a lack of tactile feedback with laparoscopic instruments, although this may be offset by improvements in instrument dexterity and visualization.11 Therefore, a technique that improves the ability of visual identification of the ureter and distinguishment of diseased tissues from a healthy ureter is very much needed. Indocyanine green (ICG) is a fluorescent dye; when it is activated by near infrared fluorescence (NIRF) light, it can visualize the desired anatomical structure in real time. As a real-time contrast agent, ICG is very suitable for intraoperative use because of its tissue penetrating ability, high signal-to-noise ratio, and excellent safety.12 ICG may facilitate improved identification of the ureter, assessment of tissue vascularity, and excision of non-viable segments. The adoption of ICG fluorescence imaging in effectiveness in complex laparoscopic gynecological surgeries and its multiple applications has increased over time. The major field of application is the evaluation at the time of anastomosis construction along with a better intraoperative identification of anatomical structures such as the ureter, urethra, lymph nodes, and tumor location. These objectives are relevant because they aim to improve patient safety by avoiding or reducing the risk of complications.Intraureteral ICG and subsequent visualisation under NIR fluorescence seem a very promising.
technique for primary and secondary prevention against iatrogenic ureteral injury.ICG NIR fluorescence imaging is a promising tool for intraoperative decision-making during different minimally invasive surgical procedures. Since the introduction of indocyanine green (ICG) as a fluorophore in nearinfrared imaging, fluorescence visualization has become an essential tool in many fields of surgery. In the field of gynecology, recent new applications have been proposed and found their place in clinical practice. Hence the present study was done at our tertiary care centre compare the role of Intraureteral injection of ICG dye with lighted ureteral stent to visualize ureter and to evaluate their feasibility and effectiveness in complex laparoscopic gynaecological surgeries.
AIMS AND OBJECTIVES
To compare the role of Intraureteral injection of ICG dye with lighted ureteral stent to visualize ureter and to evaluate their feasibility and effectiveness in complex laparoscopic gynaecological surgeries. REVIEW OF LITERATURE
Laparoscopy (from Ancient Greek λαπάρα (lapara) 'flank, side', and σκοπέω (skopeo) 'to see') is an operation performed in the abdomen or pelvis using small incisions (usually 0.5–1.5 cm) with the aid of a camera. The laparoscope aids diagnosis or therapeutic interventions with a few small cuts in the abdomen. Laparoscopic surgery, also called minimally invasive surgery (MIS), bandaid surgery, or keyhole surgery, is a modern surgical technique. There are a number of advantages to the patient with laparoscopic surgery versus an exploratory laparotomy.
History
It is difficult to credit one individual with the pioneering of the laparoscopic approach. In 1901, Georg Kelling of Dresden, Germany, performed the first laparoscopic procedure in dogs, and, in 1910, Hans Christian Jacobaeus of Sweden performed the first laparoscopic operation in humans.13 In the ensuing several decades, numerous individuals refined and popularized the approach further for laparoscopy. The advent of computer chip-based television cameras was a seminal event in the field of laparoscopy. This technological innovation provided the means to project a magnified view of the operative field onto a monitor and, at the same time, freed both the operating surgeon's hands, thereby facilitating performance of complex laparoscopic procedures. The first publication on modern diagnostic laparoscopy by Raoul Palmer appeared in 1947,14 followed by the publication of Hans Frangenheim and Kurt Semm, who both practised CO2 hysteroscopy from the mid-1970s.15 Patrick Steptoe, one of the pioneers of IVF, was important in popularizing laparoscopy in the UK. He published a textbook, Laparoscopy in Gynaecology, in 1967.16 In 1972, Clarke invented, published, patented, presented, and recorded on film laparoscopic surgery, with instruments marketed by the Ven Instrument Company of Buffalo, New York.17 In 1975, Tarasconi, from the Department of Ob-Gyn of the University of Passo Fundo Medical School (Passo Fundo, RS, Brazil), started his experience with organ resection by laparoscopy (Salpingectomy), first reported in the Third AAGL Meeting, Hyatt Regency Atlanta, November 1976 and later published in The Journal of Reproductive Medicine in 1981.16 This laparoscopic surgical procedure was the first laparoscopic organ resection reported in medical literature. In 1981, Semm, from the gynecological clinic of Kiel University, Germany, performed the first laparoscopic appendectomy. Following his lecture on laparoscopic appendectomy, the president of the German Surgical Society.
wrote to the Board of Directors of the German Gynecological Society suggesting suspension of Semm from medical practice. 15,19 In 1985, Erich Mühe, professor of surgery in Germany, performed the first laparoscopic cholecystectomy.20Afterward, laparoscopy gained rapid acceptance for non-gynecologic applications. Prior to Mühe, the only specialty performing laparoscopy on a widespread basis was gynecology, mostly for relatively short, simple procedures such as a diagnostic laparoscopy or tubal ligation. The introduction in 1990 of a laparoscopic clip applier with twenty automatically advancing clips (rather than a single load clip applier that would have to be taken out, reloaded and reintroduced for each clip application) made general surgeons more comfortable with making the leap to laparoscopic cholecystectomies (gall bladder removal). A special type of laparoscope called a fertiloscope, which is modified for transvaginal application, can be used. A dye test may be performed to detect any blockage in the reproductive tract, wherein a dark blue dye is passed up through the cervix and is followed with the laparoscope through its passage out into the fallopian tubes to the ovaries.
Robot-assisted surgery
The concept of using standard hand grips to control manipulators and cameras of various sizes down to sub-miniature was described in the Robert Heinlein story 'Waldo', which also mentioned brain surgery. The first robot to assist in surgery was the Arthrobot, which was developed and used for the first time in Vancouver in 1985. This robot assisted in being able to manipulate and position the patient's leg on voice command. Intimately involved were biomedical engineer James McEwen, Geof Auchinleck, a UBC engineering physics grad, and Dr. Brian Day as well as a team of engineering students. The robot was used in an orthopaedic surgical procedure on 12 March 1984, at the UBC Hospital in Vancouver. Over 60 arthroscopic surgical procedures were performed in the first 12 months, and a 1985 National Geographic video on industrial robots, The Robotics Revolution, featured the device. Other related robotic devices developed at the same time included a surgical scrub nurse robot, which handed operative instruments on voice command, and a medical laboratory robotic arm. A YouTube video entitled Arthrobot- the world's first surgical robot illustrates some of these in operation.In 1985 a robot, the Unimation Puma 200, was used to orient a needle for a brain biopsy while under CT guidance during a neurological procedure.22 In the late 1980s, Imperial College in London developed PROBOT, which was then used to perform prostatic surgery. The advantages to this robot was its small size, accuracy and lack of fatigue for the surgeon. In 1992, the ROBODOC was introduced and revolutionized orthopedic surgery by being able to assist with hip replacement surgeries.23The latter was the first surgical robot that was approved by the FDA in 2008.
Robotic Surgery in Gynecology
he first report of robotic surgery in gynecology was published in 1999 from the Cleveland Clinic The adoption of robotic surgery has contributed to the increase in minimally invasive surgery for gynecologic disease. Gynecologic procedures may take longer with robot-assisted surgery and the rate of complications may be higher, but there are not enough high-quality studies to know at the present time.25 In the United States, robotic-assisted hysterectomy for benign conditions was shown to be more expensive than conventional laparoscopic hysterectomy in 2015, with no difference in overall rates of complications.This includes the use of the da Vinci surgical system in benign gynecology and gynecologic oncology. Robotic surgery can be used to treat fibroids, abnormal periods, endometriosis, ovarian tumors, uterine prolapse, and female cancers.27Using the robotic system, gynecologists can perform hysterectomies, myomectomies, and lymph node biopsies.27 The Memic robotic system is aimed to provide a robotic platform for natural orifice transluminal endoscopic surgery (NOTES) for myomectomy through the vagina.
Anatomy of the ureter The ureters are bilateral thin (3 to 4 mm) tubular structures that connect the kidneys to the urinary bladder, transporting urine from the renal pelvis into the bladder. The muscular layers are responsible for the peristaltic activity that the ureter uses to move the urine from the kidneys to the bladder.
Embryologically, the ureter originates from the ureteric bud, which is a protrusion of the mesonephric duct, a part of the genitourinary system development.
The ureters begin at the ureteropelvic junction (UPJ) of the kidneys, which lie posteriorly to the renal vein and artery in the hilum[1]. The ureters then travel inferiorly inside the abdominal cavity. They pass over (anterior to) the psoas muscle and enter the bladder on the posterior bladder aspect in the trigone.
Three areas along the path of the ureter are clinically significant for renal stones lodging. These areas are: the ureteropelvic junction (UPJ), the ureterovesical junction (UVJ), and the crossover of the common iliac arteries. The UPJ is where the pelvis of the kidney transitions into the ureter and the UVJ is where the ureters enter the bladder.
The blood supply to the ureter is segmental. The upper ureter closest to the kidneys receives blood directly from the renal arteries. The middle part is supplied by the common iliac arteries, branches from the abdominal aorta, and the gonadal arteries. The most distal part of the ureter receives blood from branches of the internal iliac artery.
T12 through L2 provide innervation to the ureters, creating a ureteric plexus. Pain may refer to T12-L2 dermatomes. Due to its location, the ureter can be damaged in colon and rectal surgery and gynecologic surgeries.
Structure and Function
The ureteric wall is composed of three main of tissue: inner mucosa, middle muscle layer and outer serosa. The lining of the inner layer is transitional epithelium. Deeper to it is the lamina propria, which is combined with the epithelium make up the mucosal lining. The next deeper layer of tissue is the smooth muscle layer or lamina propria. An inner longitudinal and an outer circular layer comprise the smooth muscle layer of the ureter.The path of the ureter is along the anterior edge of the psoas muscle, which is the general area where the gonadal vessels cross anteriorly to the ureter a third of the way to the bladder. The ureter crosses over the common iliac arteries, showing the anatomical landmark of the bifurcation of the common iliac vessels into internal and externa iliac vessels. The ureters finally enter on the posterior wall of the bladder where they incorporate into the trigone[2]. The ureters have specific anatomic relationships dependent upon which side of the body. The right ureter lies in close relationship to the ascending colon, cecum, and appendix. The left ureter is close to the descending and sigmoid colon..
The nomenclature of the ureter is based on its anatomic relationship to surrounding structures. The abdominal ureter is the segment of the ureter that extends from the renal pelvis to the iliac vessels. The pelvic ureter extends from the iliac vessels to the bladder.31There is an alternative method of ureteral nomenclature: upper, middle, and lower segments. The upper ureter extends from the renal pelvis to the upper border of the sacrum. The middle ureter continues from the upper to lower borders of the sacrum. The distal ureter continues from the lower border of the sacrum to the bladder.
Blood Supply and Lymphatics
The ureters receive their blood supply from multiple arterial branches[5]. In the upper or abdominal ureter, the arterial branches stem from the renal and gonadal artery, abdominal aorta, and common iliac arteries. In the pelvic and distal ureter, the arterial branches come from the vesical and uterine arteries, which are branches of the internal iliac artery. The arterial supply will course along the ureter longitudinally creating a plexus of anastomosing vessels. This is of clinical significance because it allows for safe mobilization of the ureter during surgery when proper exposure from surrounding structures is crucial. The venous and lymphatic drainage of the ureter mirrors that of the arterial supply. The lymphatic drainage is to the internal, external, and common iliac nodes. The lymphatic drainage of the left ureter is primarily to the left paraaortic lymph nodes while the drainage of the right ureter primarily drains to the right paracaval and interaortocaval lymph nodes.The exact role of the innervation of the ureter is unclear, but the innervation for ureteral peristalsis originates from the intrinsic smooth muscular pacemaker sites. Within the renal collecting system, the minor calyces are the location for the pacemaker sites.32 There is preganglionic sympathetic input from T10 through L2. The aorticorenal, superior, and inferior hypogastric autonomic plexuses give rise to the postganglionic fibers. S2 through S4 provide parasympathetic innervation to the ureter.
Muscles
The ureter is made up of 3 layers: innermost mucosa, muscularis, and the outer adventitia. The mucosa is lined with circular transitional epithelium. The keratin in this layer is responsible for the waterproof propereties. The musclaris layer is made up of 2 longitudinal layers and a circular layer in the middle. The peristaltic motion of the ureter arises from the continuous smooth muscle layer from the ureter to the minor renal calyces, where the pacemaker for ureteric persitalsis is thought to arise The adventitia is made up of dense collagen and elastic fibers. Surgical Considerations
The most common causes of ureteral injury are iatrogenic.33The overall incidence of iatrogenic ureteral injury varies between 0.5% to 10%. The most common type of procedure responsible for iatrogenic injury to the ureter is a hysterectomy (54%) due to the proximity of the uterine artery to the distal ureter.34,35 As the ureter courses into the pelvis, it nears the infundibulo-pelvic ligament where it courses below to the uterine artery. Ureteral injuries may present with flank pain, ileus, hematuria, and prolonged high drain outputs. Elevated laboratory levels include BUN and creatinine. The close proximity of the distal ureter to the uterine vessels is the site where injuries most commonly occur during gynecologic procedures. The next most commonly injured area is at the pelvic brim, in the area of the infundibulopelvic ligament. 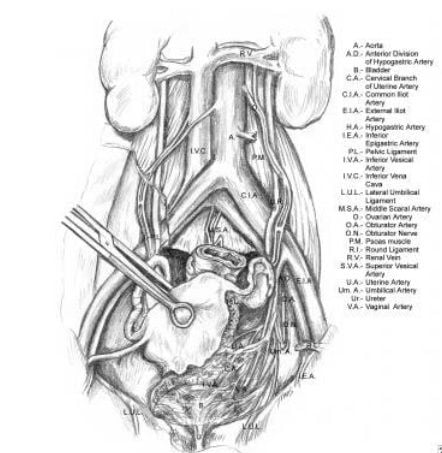
Relevant anatomy of the ureter, illustrating its course from the renal pelvis to the bladder. Note the ureter's proximity at the pelvic brim to the infundibulopelvic ligament.
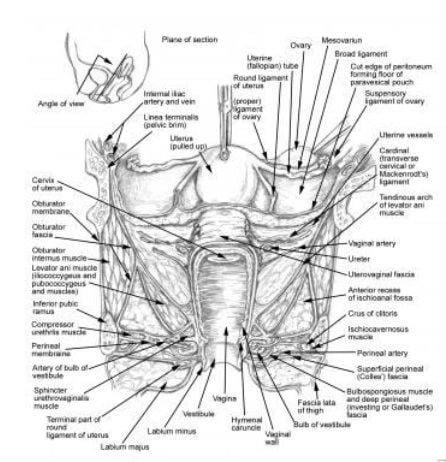
the proximity of the ureter to the uterine vessels at the level of the cervix. Most ureteral injuries following gynecologic surgery occur in this area.
Ureteral Injury
Berard (1841) and Simon (1869) reported the earliest recorded repairs of ureteral injuries in gynecologic surgery. While the exact details of this procedure are unknown, the ureter and its course were poorly understood. In the early 1900s, Dr John Sampson, then a young faculty member at Johns Hopkins University, conducted the first systematic study of the ureter. During the next 100 years, as the surgical management for gynecologic disease progressed, many contributions were made to the understanding of the etiology, prevention, diagnosis, and treatment of iatrogenic ureteral injuries.
Etiology
The 6 most common mechanisms of operative ureteral injury are as follows:
• Crushing from misapplication of a clamp
• Ligation with a suture
• Transsection (partial or complete)
• Angulation of the ureter with secondary obstruction
• Ischemia from ureteral stripping or electrocoagulation
• Resection of a segment of ureter
• Any combination of these injuries may occur. Factors that predispose a patient to iatrogenic urologic injury incllude the following: • Uterus size larger than 12 weeks' gestation
• Ovarian cysts 4 cm or larger
• Endometriosis
• Pelvic inflammatory disease
• Prior intra-abdominal surgery36
• Radiation therapy
• Advanced malignancy
• Anatomical anomalies of the urinary tract Ureteral injuries can be either expected or unexpected.
They may be the result of carelessness or due to a technically challenging procedure.
• Crushing from misapplication of a clamp
• Ligation with a suture
• Transsection (partial or complete)
• Angulation of the ureter with secondary obstruction
• Ischemia from ureteral stripping or electrocoagulation
• Resection of a segment of ureter
• Any combination of these injuries may occur. Factors that predispose a patient to iatrogenic urologic injury incllude the following: • Uterus size larger than 12 weeks' gestation
• Ovarian cysts 4 cm or larger
• Endometriosis
• Pelvic inflammatory disease
• Prior intra-abdominal surgery36
• Radiation therapy
• Advanced malignancy
• Anatomical anomalies of the urinary tract Ureteral injuries can be either expected or unexpected.
They may be the result of carelessness or due to a technically challenging procedure.
Pathophysiology
The pathophysiology of ureteral injury depends on many factors, including the type of injury and when the injury is identified. Ureteral injuries may have numerous consequences, including the following: Spontaneous resolution and healing of the injured ureter
• Hydronephrosis
• Ureteral necrosis with urinary extravasation
• Ureteral stricture formation
• Uremia
• Spontaneous resolution and healing
If the injury to the ureter is minor, easily reversible, and noticed immediately, the ureter may heal completely and without consequence. Inadvertent ligation of the ureter is an example of such an injury. If this injury is noticed in a timely fashion, the suture can be cut off the ureter without significant injury.
• Hydronephrosis
• Ureteral necrosis with urinary extravasation
• Ureteral stricture formation
• Uremia
• Spontaneous resolution and healing
If the injury to the ureter is minor, easily reversible, and noticed immediately, the ureter may heal completely and without consequence. Inadvertent ligation of the ureter is an example of such an injury. If this injury is noticed in a timely fashion, the suture can be cut off the ureter without significant injury.
Hydronephrosis
If complete ligation of the ureter occurs, the urine from the ipsilateral kidney is prevented from draining into the bladder, leading to hydronephrosis and progressive deterioration of ipsilateral kidney function. These events may occur with or without symptoms. If the urine in this obstructed system becomes infected, the patient will almost certainly become septic with pyonephrosis.
Ureteral necrosis with urinary extravasation
In complete unrecognized ligation of the ureter, a section of the ureteral wall necroses because of pressure-induced ischemia. The ischemic segment of the ureter eventually weakens, leading to urinary extravasation into the periureteral tissues. If the urinary extravasation drains into the adjacent peritoneum, urinary ascites may develop. If the urinary ascites is infected, peritonitis may ensue. If the peritoneum has remained closed, a urinoma may form in the retroperitoneum.
Ureteral stricture
Ureteral stricture may occur when the adventitial layer of the ureter is stripped or electrocoagulated. When the adventitia, the outer layer of the ureter that contains the ureteral blood supply, is disturbed by either stripping or electrocoagulation, ischemia to a particular segment of ureter may result. Ischemic strictures of the ureter may then develop, leading to obstruction and hydronephrosis of the ipsilateral kidney.
Uremia
Uremia results when ureteral injury causes total urinary obstruction. This may result from bilateral ureteral injury or from a unilateral injury occurring in a solitary functioning kidney. Anuria is the only immediate sign of imminent uremia. These cases require immediate intervention to preserve renal function.
Basics of Near-Infrared Fluorescence (NIRF) Molecular Imaging
Near-infrared fluorescence (NIRF) is an emerging translational, intravascular imaging modality that has the ability to capture a wide range of in vivo pathobiological processes. NIRF molecular imaging entails (1) injecting targeted or activatable NIRF molecular imaging agents, which consist of fluorescent conjugates (e.g., NIR fluorophores conjugated to an antibody, peptide, or small molecule) that concentrate in atheroma and bind to molecular targets, and (2) detecting the fluorophore emission signal from a NIRF catheter and console detection system.37 After injecting targeted fluorophores, excitation light from the near-infrared spectrum (650–900 nm) is directed at the arterial wall and used to stimulate fluorophores from ground state (S0) to an excited state. The excited fluorophores emit energy in the form of photons (fluorescence emission) and then return to the ground state, and are then available for further excitation. Fluorescence emission occurs at a lower energy and a longer wavelength, and this emission light is detectable with a high sensitivity charge-coupled device (CCD) camera and appropriate emission filter that attenuates the initial shorter wavelength excitation light (34). Compared to visible light range fluorescence detection, characteristics of NIRF imaging that make it a highly sensitive imaging modality include: (A) less light absorption by hemoglobin, lipid, and water, allowing deeper penetration of light into tissue; and (B) reduced background tissue autofluorescence, allowing for high signal-to-background ratio.
Near-infrared fluorescence imaging
Near-infrared fluorescence (NIRF) imaging is an emerging clinical technology that requires administration of a fluorescence-imaging agent that can be excited at near-infrared (NIR) wavelengths of ≥760nm. Upon illuminating tissue surfaces with penetrating NIR light to excite the imaging agent within the tissues, the generated fluorescence is collected to form a two-dimensional (2D) image demarking the tissue deposition of the NIRF imaging agent. While far-red and NIR light between the wavelength ranges of 690–900 nm penetrate deeply in tissues, endogenous chromophore fluorescence when excited by light of wavelengths <780 nm, creates a high autofluorescence background for molecularly targeting exogenous imaging agents in tissues. The tissue depth to which the NIRF imaging can detect NIRF imaging agents is dependent upon their brightness and the sensitivity of the device, but has been estimated to be between 3 and 4 cm beneath the tissue surfaces in intensified devices1 and <2 cm in others.40 Three-dimensional tomographic imaging using 2D projection data as well as time-dependent and independent methods has been developed for small animal imaging but, owing to these limitations in tissue penetration, has not been translated to clinical imaging.
The exciting concept of conjugating a NIR excitable fluorophore to a small molecule, protein or antibody that targets an extracellular disease marker for diagnostic, molecular imaging has been postulated for years by several investigators.41-43 The use of NIRF imaging for molecularly guided surgical resection of cancers could dramatically reduce residual tumour burden as well as surgical morbidity associated with excising sufficient tissues to avoid having positive surgical margins. However, the tissue depth, concentration and dose at which a “first-in-humans” imaging agent can be detected in tissues depends upon several factors but, most importantly, upon the sensitivity of the imaging device. Unlike positron emission tomography, scintigraphy, single-photon emission and the γ probe used to detect radiolabelled molecular targeting agents for diagnostic imaging and intraoperative detection, fluorescence imaging devices do not have phantoms and standards to assess performance metrics, and there are no traceable standards to quantify or compare performance between fluorescence imaging devices.
NIR-excited fluorophore used clinically is indocyanine green (ICG). Since 1956, ICG has been approved by the US Food and Drug Administration for intravenous (i.v.) administration at a concentration of 2.5 mgml−1 with doses of up to 25mg in adults, 12.5mg in children and 6.25mg in infants. ICG has been used in the clinic as a reagent for determining cardiac output, hepatic function and ophthalmic angiography. It has an excellent record of safety, and there is no demonstrable evidence of phototoxicity associated with its use. After administration, ICG binds tightly to plasma proteins and has a half-life of several minutes in blood circulation, which allows repeated intraoperative i.v. administration for fluorescence angiography. In the plasma, the absorption and emission peaks of ICG are shifted towards longer wavelengths, to around 807 and 822nm,10 respectively, but still reside in the “optical window” of the tissues. Compared with other NIR-excited fluorophores, the quantum efficiency (QE) of ICG is low and reported to be 0.02 at 780 nm excitation and 830 nm emission;44 it is comparatively unstable once reconstituted in saline; and it has no functional group for conjugation to compound for molecular imaging. Using ICG as a non-specific blood vascular imaging agent, NIRF angiography has been used intraoperatively in coronary, neurosurgical and vascular surgeries8 as well for non-invasive assessment of superficial perfusion.
Most recently, ICG has been used in off-label intradermal and subcutaneous administrations at varying doses for evaluating the lymphatic circulation to identify sentinel lymph nodes (SLNs) in surgical oncology, assess lymphovenous anastomoses (LVA) surgery46 and non-invasively map the lymphatic vasculature. Indeed, “ICG lymphography” has been found to be superior to lymphoscintigraphy for diagnostic imaging of early lymphoedema in upper extremities20 and enables early diagnosis of lymphoedema before the onset of symptoms. Yet, the doses of ICG and the design of devices used in these and other clinical studies vary widely, suggesting variable device performance. Although the architecture can be dramatically different among these devices, the core components are (i) the light source for exciting ICG; (ii) optical filters for separating emitted fluorescent signals from strong backscattered excitation light and ambient light signals; and (iii) an area detector for sensing the emitted fluorescent signals. Undoubtedly, the performance of a device is ultimately determined by these core components. requiring different dosages of ICG ranging from micrograms to milligrams per injection for visualizing the lymphatics.
The exciting concept of conjugating a NIR excitable fluorophore to a small molecule, protein or antibody that targets an extracellular disease marker for diagnostic, molecular imaging has been postulated for years by several investigators.41-43 The use of NIRF imaging for molecularly guided surgical resection of cancers could dramatically reduce residual tumour burden as well as surgical morbidity associated with excising sufficient tissues to avoid having positive surgical margins. However, the tissue depth, concentration and dose at which a “first-in-humans” imaging agent can be detected in tissues depends upon several factors but, most importantly, upon the sensitivity of the imaging device. Unlike positron emission tomography, scintigraphy, single-photon emission and the γ probe used to detect radiolabelled molecular targeting agents for diagnostic imaging and intraoperative detection, fluorescence imaging devices do not have phantoms and standards to assess performance metrics, and there are no traceable standards to quantify or compare performance between fluorescence imaging devices.
NIR-excited fluorophore used clinically is indocyanine green (ICG). Since 1956, ICG has been approved by the US Food and Drug Administration for intravenous (i.v.) administration at a concentration of 2.5 mgml−1 with doses of up to 25mg in adults, 12.5mg in children and 6.25mg in infants. ICG has been used in the clinic as a reagent for determining cardiac output, hepatic function and ophthalmic angiography. It has an excellent record of safety, and there is no demonstrable evidence of phototoxicity associated with its use. After administration, ICG binds tightly to plasma proteins and has a half-life of several minutes in blood circulation, which allows repeated intraoperative i.v. administration for fluorescence angiography. In the plasma, the absorption and emission peaks of ICG are shifted towards longer wavelengths, to around 807 and 822nm,10 respectively, but still reside in the “optical window” of the tissues. Compared with other NIR-excited fluorophores, the quantum efficiency (QE) of ICG is low and reported to be 0.02 at 780 nm excitation and 830 nm emission;44 it is comparatively unstable once reconstituted in saline; and it has no functional group for conjugation to compound for molecular imaging. Using ICG as a non-specific blood vascular imaging agent, NIRF angiography has been used intraoperatively in coronary, neurosurgical and vascular surgeries8 as well for non-invasive assessment of superficial perfusion.
Most recently, ICG has been used in off-label intradermal and subcutaneous administrations at varying doses for evaluating the lymphatic circulation to identify sentinel lymph nodes (SLNs) in surgical oncology, assess lymphovenous anastomoses (LVA) surgery46 and non-invasively map the lymphatic vasculature. Indeed, “ICG lymphography” has been found to be superior to lymphoscintigraphy for diagnostic imaging of early lymphoedema in upper extremities20 and enables early diagnosis of lymphoedema before the onset of symptoms. Yet, the doses of ICG and the design of devices used in these and other clinical studies vary widely, suggesting variable device performance. Although the architecture can be dramatically different among these devices, the core components are (i) the light source for exciting ICG; (ii) optical filters for separating emitted fluorescent signals from strong backscattered excitation light and ambient light signals; and (iii) an area detector for sensing the emitted fluorescent signals. Undoubtedly, the performance of a device is ultimately determined by these core components. requiring different dosages of ICG ranging from micrograms to milligrams per injection for visualizing the lymphatics.
Instrumentation
ICG fluorescence imaging systems incident light sources used to excite ICG; the optics that allow for collection of ICG fluorescence and rejection of ambient and backscattered incident light; and the area detector used to register the collected light.
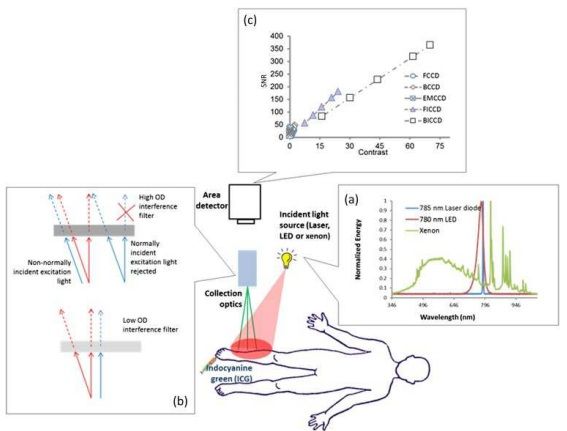

Schematic of indocyanine green (ICG)-based near-infrared fluorescence imaging system consisting of the incident light source, collection optics and area detector. (a) Represents the spectra of 785-nm laser diode, 780-nm light-emitting diode (LED) and xenon lamp, showing the laser diode with narrow bandwidth, the LED with relatively wide bandwidth and xenon with very broad bandwidth, and (b) the focus lens and emission filter. (c) A plot of signal-to-noise ratio (SNR) vs contrast for different charge-coupled device (CCD)-based detectors with integration time of 200 ms. Both front-illuminated intensified CCD (FICCD) and backilluminated intensified CCD (BICCD) are superior to the other types of CCD cameras (Zhu et al25). BCCD, back-illuminated CCD; EMCCD, electron-multiplying CCD; FCCD, frontilluminated CCD; OD, optical density.
Incident light sources for collection of indocyanine green fluorescence
The commonly used excitation light sources in ICG fluorescence imaging systems in order of increasing spectral bandwidth are (i) laser diodes; (ii) lightemitting diodes (LEDs); and (iii) filtered lamp sources, which have typical spectra. Because rejection of backscattered excitation light is performed spectrally through the use of interference filters, laser diodes enable the greatest sensitivity, since the “background” arising from “leakage” of backscattered excitation light is the lowest. By contrast, LEDs generate a broader band of wavelengths with relatively lower power output, requiring tens of LEDs integrated together for milliwatts per square centimetre of incident light. For the filtered lamp sources, the lamp sources are filtered to generate excitation light with a narrow band, but the generated excessive heat needs to be dissipated to extend the lifetime of the filter. In addition, the filtered lamp sources have low efficiencies, making it difficult to couple into an optical fibre. Hence, laser diodes and LEDs are widely adopted in the ICG fluorescence imaging systems used clinically.
Collection optics
Without exception, all ICG fluorescence imaging systems have detection sensitivities that are limited by background signals (or noise floor) that arise from one or more of the following: (i) the spectral overlap between the backscattered excitation light and collected ICG fluorescence allowing nonfluorescent signals to be registered as fluorescence; (ii) a “blue shifting” of optical filters that allows passage of non-collimated, backscattered excitation light not normally incident on the filter surface, and (iii) the limited optical density of optical filters that allows passage of a small amount of ambient and excitation light that is still significant compared with the weak, collected ICG fluorescence.47 If light that does not originate from the fluorescent dye resident in the tissues is collected, then it represents the “noise floor” making it less probable that the device can image small quantities of a NIRF agent in tissues.
Area detectors
Currently, charge-coupled device (CCD) detectors are primarily used in ICG fluorescence imaging systems and depend upon integrating the collected photons over millisecond time frames. The CCD detectors used can be divided into (i) front- and back-illuminated CCDs (FCCDs and BCCDs), (ii) electron-multiplying CCDs (EMCCDs) and (iv) intensified CCDs (ICCD), including front- and back- illuminated ICCD based on their configurations. Most imaging systems employed in humans use either FCCD or BCCDs. FCCDs are constructed in a fashion similar to the human eye by orienting polysilicon gates at the front, wiring in the middle and photodiodes for light collection at the back. This configuration blocks incident light from reaching the photodiodes resulting in relatively low QE. BCCDs contain the same elements, but by rearranging the gate structure to the back of the photosensitive area of the CCDs and by reducing the thickness of the silicon layer via proprietary etching techniques, BCCDs have more than two-fold improvement in QE than do their front-illuminated counterparts. Unlike a conventional CCD, an EMCCD has an additional electron-emitting register installed between the normal serial register and the output amplifier to multiply weak signals. The EM register is split up into several hundred stages, and each stage acts as an avalanche diode to multiply the signal. The degree of multiplication gain can be controlled by varying the clock voltages applied to the EM register.
Indocyanine Green Imaging Of Lymph Nodes for Cancer Staging
Lymph node mapping for identification of tumour draining lymph nodes for resection and subsequent pathological application in many cancers is commonly performed intraoperatively using blue dyes and/or transcutaneously and intraoperatively with non-specific technetium-99m (99mTc) radiocolloid. While emerging technologies employ i.v. administration of ultrasmall superparamagnetic iron oxide particle48 and intradermally administered radiolabelled mannose sugar, tilmanocept,49 intradermal and subcutaneous administration of ICG to locate draining lymph nodes represents an emerging area in nodal staging. The primary advantages of using a radiolabelled fluorescent molecule or NIR active compound is the direct relationship between the regions of fluorescence emitted from tissue surfaces within the surgical FOV.
Sentinel lymph node mapping in breast cancer
It is first utilized the fluorescence properties of ICG for SLN detection in patients with early breast cancer using the PDE prototype device using 25mg of ICG in 5ml of water. Intraoperatively, sentinel nodes were found by visually following the green-stained lymphatic vessels to the first-draining SLNs that were fluorescent.
Lymphatic Imaging
NIRF imaging has been used to characterize lymphoedema of the upper and lower extremities using both PDE2,and FDPM devices.Interpretation of NIRF images include aberrant lymphatic vasculature as seen comparatively to normal lymphatic vasculature.
Urethra Visualization during Transanal Tme
TaTME is a relatively new procedure50 for the curative resection of rectal tumors. It was developed to overcome the difficult dissection at the lower third of the rectum, especially in obese male patients and/or bulky tumors. Some retrospective series have reported that enhanced visualization of the dissection plane allowed better nerve preservation, improved resection margins, and improved functional outcomes compared with laparoscopic TME.51-52 However, the bottom-up transanal approach is not without complications. Incidence of iatrogenic urethral injuries has been reported, ranging from 1% to 6.7% during TaTME procedures. An international inquiry reported 34 urethral injuries from 32 surgical teams worldwide between 2010 and 2017, resulting in a significant postoperative morbidity rate of 26%. However, there is still concern that urologic injuries during TaTME may be underreported and that their incidence might be related to surgeon experience. Therefore, enhancement of urethral visualization should be considered useful and advantageous in the early learning experience. Several bioimaging modalities exist that can improve urethral identification, including ICG NIR fluorescence. Different systems have been successfully used to detect the urethra by ICG fluorescence imaging such as the IRIS ureteral kit or the PINPOINT laparoscopic system with intraurethral ICG injection or infiltration adjacent to the catheter in the urethra, respectively. Experimental studies demonstrated that direct ICG instillation into the urethra or through a urine catheter for NIR fluorescence imaging seem to be easily applicable and clinically reproducible during TaTME. Although an open-label clinical feasibility study with intraoperative direct instillation of ICG into the urethra for low rectal cancers was terminated because of technique failures, implementation of the technique has been described by some authors. Barnes et al53 evaluated the efficacy of two novel methods in cadaveric models. In the first, ICG mixed with silicone was infiltrated into 10-Fr one-way Foley catheter and allowed to set for 1 wk. In the second, new preclinical IRDye 800BK was infiltrated directly into the urethra via the urethral meatus prior to dissection. Both methods were effective in identifying the fluorescence located only within the urethra. IRDye 800BK provided a greater depth of penetration than the ICG-silicone mix, suggesting it could be a more satisfactory alternative to ICG. In addition, Barberio et al demonstrated the superior brightness of nearinfrared coating of equipment (NICE) coated catheter compared with ICGbased solutions in cadaveric experiments by exhibiting a higher fluorescence intensity than urinary catheters filled with ICG. In conclusion, no final specific recommendations can be drawn from the clinical use of ICG fluorescence imaging to identify and prevent urethral injuries during TaTME procedures.
Ureter Identification
The incidence of iatrogenic urethral injuries (IUI) ranges from 0.24% to 1.95% in colorectal surgery, and rectal cancer is considered a risk factor for IUI because of the close proximity of the ureters to the dissection plane, similar to the risk with deep pelvic endometriosis.54-55 Despite its low incidence, IUI significantly affects postoperative morbidity, mortality, length of stay and hospital charges. Visualization of the ureters is thus advocated during pelvic surgery by the visible peristalsis that occurs when the ureter is gently pressed (Kelly’s sign). However, adhesions, obesity, and an incorrect plane of dissection contribute to the lack of or incorrect recognition of the ureter, which can jeopardize its integrity. For that reason, a selective use of prophylactic urethral stents in high risk procedures is commonly accepted, but there is no sufficient evidence to support a decrease in IUI or intraoperative identification. In that setting, interest in fluorescence imaging has been increasing over time. While contrasting results were found for intravenous administration of methylene blue dye to urethral detection in colorectal surgery, ICG FA proved to be a viable alternative to real-time ureter identification and IUI prevention. Before surgery, a 6-Fr catheter is placed into the urethral orifice by cystoscopy. As ICG binds to the proteins of the ureteric epithelium,56 a retrograde injection of 5 mg ICG diluted in 2 mL of distilled water is made, and infrared emission is captured by the filtered lens system and electronically converted into green color visualizing ureter location. The technique has proven to be safe and helpful to identify the ureter in several small case-series who underwent minimally invasive pelvic surgery. As a catheter insertion of only 1 cm is required, there is a lower risk of IUI during catheterization than during conventional endoscopic stenting procedures, thus avoiding additional cystoscopies to remove the catheter. Furthermore, ICG urethral instillation is less expensive than other fluorescence-based systems such as illuminated catheters.
White et al58 recently evaluated the safety and efficacy of intraurethral ICG FA along with any potential benefit related to the technique during colorectal robotic surgery. In their experience involving 16 patients, there were short procedure times, low morbidity, and reliable urethral identification and avoidance. The United States Food and Drug Administration approval of ICG is limited to intravenous use.59Therefore, disclosure of intraurethral off-label use would be needed. In contrast, new intravenous fluorescent dyes with renal clearance, such as fluorescein sodium and IRDye® 800-BK have been used in experimental models to test the penetration of fluorescence in the ureters, with promising results for surgical practice. Additionally, the formulation of ICG in a liposome-based delivery system allows its excretion in urine in animal models and seems a promising fluorophore solution. In conclusion, evidence supporting the use of intraurethral ICG instillation in order to improve intraoperative ureter detection is based on few noncomparative feasibility studies involving mixed pelvic surgeries. Despite the efficacy demonstrated in ordinary or complex situations, no study exclusively focused on rectal cancer resections exists to date. It remains to be proven whether this innovation significantly affects surgical procedures and provides clinical benefits by reducing IUI.
White et al58 recently evaluated the safety and efficacy of intraurethral ICG FA along with any potential benefit related to the technique during colorectal robotic surgery. In their experience involving 16 patients, there were short procedure times, low morbidity, and reliable urethral identification and avoidance. The United States Food and Drug Administration approval of ICG is limited to intravenous use.59Therefore, disclosure of intraurethral off-label use would be needed. In contrast, new intravenous fluorescent dyes with renal clearance, such as fluorescein sodium and IRDye® 800-BK have been used in experimental models to test the penetration of fluorescence in the ureters, with promising results for surgical practice. Additionally, the formulation of ICG in a liposome-based delivery system allows its excretion in urine in animal models and seems a promising fluorophore solution. In conclusion, evidence supporting the use of intraurethral ICG instillation in order to improve intraoperative ureter detection is based on few noncomparative feasibility studies involving mixed pelvic surgeries. Despite the efficacy demonstrated in ordinary or complex situations, no study exclusively focused on rectal cancer resections exists to date. It remains to be proven whether this innovation significantly affects surgical procedures and provides clinical benefits by reducing IUI.
Lymph Node Mapping
Lateral pelvic lymph node dissection (LLND) allows the removal of the nodal compartment along the common iliac, internal iliac, and obturator arteries. The lymphatic stations are considered a major cause of locoregional recurrence in rectal cancer and are treated with preoperative chemoradiotherapy and curative resection.60 While it is widely accepted to perform LLND in selected patients with rectal cancer and lateral lymph nodes that are clinically positive, the Japanese Society for Cancer of the Colon and Rectum guidelines recommend LLND even when lateral lymph node metastasis is not detected by preoperative or intraoperative diagnosis. Indeed, LLND is associated with a lower rate of local recurrence compared with TME alone despite no significant differences in either overall survival or local recurrence-free survival.61 As ICG fluorescence imaging has proven to be a useful tool for identifying lymphatic drainage in colorectal surger, ICG-enhanced NIR fluorescenceguided imaging has been used to improve the accuracy and the completeness of LLND. In such cases, ICG fluorescence imaging is carried out the injection of ICG dye into the submucosal layer on the distal side of the tumor through the anus immediately before surgery. In a comparative retrospective series of 42 mid and low rectal cancer patients, the ICG group experienced a significantly lower intraoperative blood loss and a larger number of harvested lateral pelvic lymph nodes. The use of ICG may improve the safety of LLND that is affected by the technical difficulties of the procedure, complicated pelvic wall anatomy, and the effects of preoperative radiation on the tissues. In that setting, real-time identification of lateral pelvic nodes could help to distinguish lymphatic tissue from vascular and nervous structures, thus avoiding postoperative genitourinary dysfunction and providing better surgical staging. However, evidence is still limited and additional studies are needed to address the real clinical advantages and standardization of this technique.
A sentinel node (SN) is defined as the first node in the regional peritumoral area that drains the tumor. SN biopsy, in addition to conventional resection, may add clinically significant prognostic information in colorectal surgery. NIR laparoscopy with ICG mapping allowed easy intraoperative identification of mesocolic lymphatic drainage and SN during colorectal oncologic resections. Similarly Noura et al62 described the detection of SN by ICG with an NIR system in 25 patients who had no preoperative diagnosis of metastatic lateral pelvic lymph nodes. The success rate of detecting the lateral SN was 92%, and 100% concordance was observed between SN and dissected lateral lymph nodes status. That preliminary study highlighted the feasibility and reliability of lateral SN biopsy as a potential discriminator to perform LLND, but the sensitivity may be compromised by preoperative neoadjuvant chemoradiotherapy.
A sentinel node (SN) is defined as the first node in the regional peritumoral area that drains the tumor. SN biopsy, in addition to conventional resection, may add clinically significant prognostic information in colorectal surgery. NIR laparoscopy with ICG mapping allowed easy intraoperative identification of mesocolic lymphatic drainage and SN during colorectal oncologic resections. Similarly Noura et al62 described the detection of SN by ICG with an NIR system in 25 patients who had no preoperative diagnosis of metastatic lateral pelvic lymph nodes. The success rate of detecting the lateral SN was 92%, and 100% concordance was observed between SN and dissected lateral lymph nodes status. That preliminary study highlighted the feasibility and reliability of lateral SN biopsy as a potential discriminator to perform LLND, but the sensitivity may be compromised by preoperative neoadjuvant chemoradiotherapy.
Tumor Localization
Several reports have described the intraoperative identification of colonic tumors by NIR with ICG fluorescence imaging, with satisfactory results[95-98]. Accurate identification of the location of colorectal tumors is crucial in minimally invasive surgery because of the lack of tactile perception, especially for cancer at an early stage because of its small size or location on a movable part of the colon. As for rectal cancer, precise tumor site localization allows achieving a clear and safe distal resection margin, which may affect not only oncological outcomes but also bowel function and quality of life. In that setting, endoscopic tattooing of rectal tumors, both with a high-definition fluorescence imaging system (Karl Storz GmbH & Co. KG, Tuttlingen, Germany) and the PINPOINT® endoscopic fluorescence imaging system (PINPOINT system; Novadaq Technologies Inc., Mississauga, ON, Canada), is feasible and has clinical advantages. In a comparative retrospective series, 342 patients scheduled for laparoscopic colorectal resection were enrolled after propensity score matching. The tumor was tattooed in 114 patients. In a subgroup analysis of 160 patients who underwent anterior resection, the tattooed group had a significantly shorter operative time (unlike right and left colectomy), less blood loss, and a shorter hospital stay than the non-tattooed group. In addition, Goo et al compared 200 tattooed colorectal cancer patients (44 rectal cancers) with 879 non-tattooed patients (300 rectal cancers) to evaluate the effect of preoperative colonoscopic tattooing with ICG on adequate lymph node harvest in colorectal cancer. They found that preoperative tattooing in T1 colorectal cancer significantly improved adequate lymph node harvest, with a higher number of retrieved lymph nodes in rectal cancer than in colon cancer.
Fluorescence technology to localize rectal tumors has been developed not only in the field of imaging systems, but also by ICG formulation. Fenestrated peritumoral capillaries and impaired lymphatic drainage delay the washout of large molecules from tumors, which has been described as the enhanced permeability and retention effect.65 The formulation of ICG as a liposomebased delivery system improved tumor-specific localization in experimental models, with the advantages of intravenous injection and better results than free ICG.66-67 Finally, there are limited data on the role of ICG in the detection of peritoneal carcinomatosis of colorectal origin. Cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy is the only potentially curative option in patients with limited peritoneal metastases.68 Intraoperative injection of ICG seems a useful tool to identify peritoneal metastases and detect additional subclinical malignant peritoneal nodules, resulting in modification of the planned surgery in 29% of patients.
Fluorescence technology to localize rectal tumors has been developed not only in the field of imaging systems, but also by ICG formulation. Fenestrated peritumoral capillaries and impaired lymphatic drainage delay the washout of large molecules from tumors, which has been described as the enhanced permeability and retention effect.65 The formulation of ICG as a liposomebased delivery system improved tumor-specific localization in experimental models, with the advantages of intravenous injection and better results than free ICG.66-67 Finally, there are limited data on the role of ICG in the detection of peritoneal carcinomatosis of colorectal origin. Cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy is the only potentially curative option in patients with limited peritoneal metastases.68 Intraoperative injection of ICG seems a useful tool to identify peritoneal metastases and detect additional subclinical malignant peritoneal nodules, resulting in modification of the planned surgery in 29% of patients.
Fluorescence Applications in Gynecology
In 2005, when Kitai et al. introduced fluorescence-guided surgery (FGS) for breast cancer sentinel node biopsy using indocyanine green (ICG), a new era of image-guided surgery69 was initiated. Some new systems can even merge these fluorescent images with white light images and help to better orientate intraoperatively. Currently, there are only five FGS contrast agents approved for clinical use by the American Food and Drug Administration (FDA), as well as by European Medicines Agency (EMA), i.e., fluorescein, ICG, methylene blue, and 5-alanine.
Sentinel Node Biopsy
In gynecologic malignancies, the concept of SNB is not new. Historically, vulvar cancer was one of the first malignancies for which this concept was evaluated. In endometrial cancer, the most common gynecologic malignancy, two randomized clinical trials investigated the efficacy of this technique. However, these trials did not support the hypothesis of improved survival after lymphadenectomy in the early stage of the disease.
Endometrial Cancer
Even though radiocolloid and blue dye are the most commonly used tracers for SNB in a majority of cancers based on a recent survey among American gynecologic oncologists, ICG was most commonly used for SNB in endometrial cancer (97.3% of the responders) and in cervical cancer (92.5%). The first application of ICG for the SNB procedure in endometrial cancer was published by Furukawa et al., in which fluorescent nodes were detected in 83% of patients, with all cases found bilaterally.72 No false-negative nodes were found. The first minimally invasive SNB in endometrial cancer was published by Rossi et al., with the detection of sentinel nodes in 85% of cases (17 patients).73 In 60% of cases, positive nodes were found bilaterally, with no false-negative cases. In a study that compared ICG with isosulfan blue (IB), the nodes were found with the naked eye in 77% of cases with IB and in 97% of cases when using the fluorescent properties of ICG.
Cervical Cancer
The first experience in cervical cancer sentinel node biopsy using ICG was published by Crane et al., in which a mixture of ICG and patent blue was injected for lymph node staining.74 SNB fluorescence was observed in 60% of cases (6 patients). Once pelvic lymphadenectomy was performed, additional nodes (11 ones) showed fluorescence with one fluorescent node harboring metastases. No false-negative results were found, and SNBs were found bilaterally in half of the cases. The detection rate in tumors below 2 cm was 80% and only 40% in larger tumors.
An application of this procedure in cervical cancer is of the highest importance since up to 20% of patients with early cervical cancer present with lymph node metastases. The accurate detection of nodal disease impacts 5-year survival, which decreases from 92 to 64% for those with lymph node metastases. The application of sentinel node biopsy may decrease the long-term morbidity rate related to lymphadenectomy that occurs in up to 20% of those undergoing the more radical procedure.
An application of this procedure in cervical cancer is of the highest importance since up to 20% of patients with early cervical cancer present with lymph node metastases. The accurate detection of nodal disease impacts 5-year survival, which decreases from 92 to 64% for those with lymph node metastases. The application of sentinel node biopsy may decrease the long-term morbidity rate related to lymphadenectomy that occurs in up to 20% of those undergoing the more radical procedure.
Vulvar Cancer
The first description of an ICG use in vulvar cancer SNB was presented by Crane et al. in 10 patients and showed that this technique was feasible but only in patients with a BMI below 25 kg/m2. It was associated with limited penetration of fluorescence through extensive adipose tissue in the groin. This issue was also highlighted in a publication by Prader et al., in which the authors also found that in the group with BMI > 30 kg/m2, sentinel nodes were found in 93.3% of patients when radiocolloid was used and in 86.7% of patients in the ICG group. In a publication by Verbeek et al., if ICG was mixed with radiocolloid in 12 patients with early stage vulvar cancer, the detection rate of sentinel nodes was 100%
Ovarian Cancer
In the systematic review from 2019, 10 articles were analyzed with a detection rate of 90.3%. Concerning ICG and radiocolloid in seven ovarian cancer patients, the detection rate was 100%. In a series of five patients using only ICG, sentinel nodes were detected in all cases.
Vaginal Cancer
In this malignancy, only a case report presenting a successful sentinel node mapping in vaginal cancer is available. ICG was injected into the bilateral side of the tumor. Sentinel nodes were found bilaterally in the obturator fossa.
Multi-Channel Fluorescence
The issue of the injection site in uterine cancer or of the cervical injection in endometrial cancer is still debatable. Sentinel nodes might be visible using two fluorophores during the same operation. In a study by Laios et al., the fluorescence properties of methylene blue were used. Methylene blue and ICG were visible in two patients sharing common lymphatic structures no matter the injection site.77In one case, the lymph nodes were stained with both fluorophores. However, in the second case, the true positive para-aortic sentinel node was only stained with ICG after uterine fundus injection but not with MB after cervical injection. Similar multispectral fluorescence imaging during prostatectomy was proposed using fluorescein and ICG mixed with radiocolloid. Undoubtedly, multispectral image-guided surgery will soon be a perfect tool to differentiate anatomical structures from different fluorophores.
Mesometrium
Enhancements in surgical techniques that will help to improve oncologic outcomes are among the key objectives of any surgical research. Total mesorectal excision (TME) proposed by RJ Heald revolutionized the surgical technique for several cancers, as well as the concept of embryological planes. This led to an improved local control after rectal cancer surgery.78This idea was also adopted in other organ-specific surgical techniques. Embryologically based compartmental surgery was also proposed by Höckel et al., in the treatment of gynecologic cancer.
Fluorescent Angiography Uterine Tube Perfusion
The concept of uterine transplantation is emerging, although it is still in its infancy period. During donor hysterectomy, normally, both uterine tubes are transected. In the case of pregnancy, the recipient of the uterus has to undergo an in vitro fertilization (IVF). It is hypothesized that transplantation made together with the uterine tubes may facilitate a spontaneous conception. In the study by Farag et al., they investigated ex vivo and in vivo relative fluorescence, as well as the fluorescence intensity ratio,79 using ICG angiography. Vascular perfusion for uterine tubes originates from uteroovarian vasculature alone. This is especially important since a less extensive dissection for bilateral arterial and veins is currently proposed during transplantation. Additionally, the authors found differences in the location of utero-ovarian vessels. The length of utero-ovarian vessels may also help with an easier re-anastomosis and a safer surgery. This study may pave the way for complex uterus and attached uterine tubes transplantation, based on a tailored separation of utero-ovarian vasculature with fluorescence guidance, and facilitate a spontaneous conception.
Ureteral Branch of Uterine Artery Detection
The surgical treatment of cervical cancer requires an extensive conventional radical hysterectomy, which is sometimes associated with a poor blood supply of the ureters postoperatively. This may lead to complex postoperative complications such as ischemic necrosis of the ureter, urinary fistula, or stenosis of the ureter. Possible prevention of such complications might be proposed by preserving the ureteral branch of the uterine artery. A report of two cases with ureteral branch-sparing hysterectomy was presented using fluorescence angiography for the identification of a ureteral branch Additionally, with this fluorescence angiography technique, the authors also evaluated the perfusion of the uterine artery and ureter. No postoperative complications related to the ureter vasculature were reported.
Vaginal Cuff Angiography
Vaginal cuff dehiscence represents a severe complication after hysterectomy. The implementation of robotic surgery increased the rate of this complication, with a rate of 0.6% for abdominal hysterectomy and up to 3.16% after robotic hysterectomy. Vaginal cuff angiography using ICG was performed after robotic hysterectomy in 20 patients. No difference in terms of vaginal cuff dehiscence prevention was observed for monopolar or ultrasonic devices. Only longer instrument activation times in the open cuff showed a decreased cuff perfusion. A similar study was performed for laparoscopic hysterectomy . The added value of ICG guidance to vaginal cuff angiography and flap reconstructing is improving the technique, as it is now used in daily practice. Then, another point is that it helps to decrease the morbidity rates.
Flap Reconstruction
Postoperative complications after bilateral groin lymphadenectomy is not a rare situation. However, a wound breakdown is a difficult complication to treat. In the literature, a case was reported with such a complication, and a pedunculated left anterolateral thigh flap was presented. A fluorescence angiography with ICG for flap viability was performed without any further complications associated with flap healing. Two months after the operation, the patient received radiotherapy.
Uterus Transplantation
From a surgical standpoint, the key to successful uterus transplantation is the quality of vascular anastomoses. An occlusion of anastomoses is complex and might be associated with inadequate anticoagulation, poor graft fixation, low immunosuppression, inadequate surgical technique, patient’s age, and mutations associated with venous thromboembolism. Kengelbach et al. used different techniques in a sheep model for intraoperative and postoperative blood flow measurements using ICG, as well as Doppler flowmetry.
Trachelectomy and Uterine Artery Angiography
Trachelectomy is performed in early stage cervical cancer among patients desiring future fertility. There is some extensive literature evidence regarding its safety, as well as good outcomes associated with 16 to 23% of the pregnancy rate. However, one of the questions associated with surgical steps is the preservation of the uterine artery during this operation. In the group of 20 patients, half of them underwent uterine artery sparing trachelectomy, and the second half, uterine n