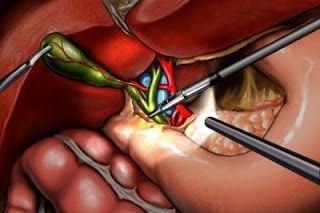
Bleeding Complications Following Laparoscopic Cholecystectomy
Protocol For Submission Of Thesis For The Award Of Masters In Minimal Access Surgery.
The Global Open University Of Nagaland, World Laparoscopy Hospital Centre, Cyber City, Gurgoan, Delhi Ncr, India.
Certification :
We have participated and supervised this research done by Dr. Isma’il Abdulkadir Jibril Tsauni on “Bleeding Complications Following Laparoscopic Cholecystectomy” conducted in our centre.
Dr. J S Chowhan
MBBS, MS, F.MAS, PGDHHM.
COORDINATOR
-----------------------------------------------------------------
Dr. (PROF.) RK Mishra
MBBS, MS, MWALS, MRCS, M. MAS, F. MAS, FICRS, Ph D.
Professor and Head, Minimal Access Surgery.
TGO University Of Nagaland And Medical Director,
World Laparoscopy Hospital,X100 , Cyber City, DLF Phase 2 , Gurgaon, Haryana, India.
-------------------------------------------------------------
Dr. Isma’il Abdulkadir Jibril Tsauni
Department Of Surgery, Aminu KanoTeaching Hospital, Kano State, Nigeria.
Declaration :
Dr. Isma’il Abdulkadir Jibril Tsauni
Department Of Surgery, Aminu Kano Teaching Hospital, Kano State, Nigeria.
Acknowledgement :
Introduction :
The Global Open University Of Nagaland, World Laparoscopy Hospital Centre, Cyber City, Gurgoan, Delhi Ncr, India.
Certification :
We have participated and supervised this research done by Dr. Isma’il Abdulkadir Jibril Tsauni on “Bleeding Complications Following Laparoscopic Cholecystectomy” conducted in our centre.
Dr. J S Chowhan
MBBS, MS, F.MAS, PGDHHM.
COORDINATOR
-----------------------------------------------------------------
Dr. (PROF.) RK Mishra
MBBS, MS, MWALS, MRCS, M. MAS, F. MAS, FICRS, Ph D.
Professor and Head, Minimal Access Surgery.
TGO University Of Nagaland And Medical Director,
World Laparoscopy Hospital,X100 , Cyber City, DLF Phase 2 , Gurgaon, Haryana, India.
-------------------------------------------------------------
Dr. Isma’il Abdulkadir Jibril Tsauni
Department Of Surgery, Aminu KanoTeaching Hospital, Kano State, Nigeria.
Declaration :
I Dr. Isma’il Abdulkadir Jibril Tsauni hereby declare that this thesis titled “ Bleeding Complications In Laparoscopic Cholecystectomy” has not been submitted in candidature for any other degree and I have no objection for this thesis to be copied in part or whole, or used for research purposes.
Dr. Isma’il Abdulkadir Jibril Tsauni
Department Of Surgery, Aminu Kano Teaching Hospital, Kano State, Nigeria.
Acknowledgement :
I give gratitude to Allah SWT for a successful completion of the Masters programme. My sincere thanks to Dr(Prof) RK Mishra and Wife Mrs Sadhana for giving me a home away from home, Dr. JS Chowhan for his tireless support,to the entire crew of the World Laparoscopy Hospital, Dr. Parveen Bhatia( Sir Gan Garam Hospital), Dr. Arun Prasad(Apollo Hospital), Dr. Adarsh Chaudhary ( Medanta Hospital) for support and guide during my research work.
My appreciation to the Department Of Surgery and Management Of Aminu Kano Teaching Hospital for their support and assistance.
My appreciation to the Department Of Surgery and Management Of Aminu Kano Teaching Hospital for their support and assistance.
Introduction :
Since it’s introduction into the surgeon’s armamentarium, laparoscopic cholecystectomy (LC) has become the operation of choice for patients cholelithiasis, as it is associated with lesser postoperative pain and discomfort, better cosmesis, shorter hospital stay and a chance for early return to work. However, on occasion, the procedure can be associated with potentially life threatening complications; these may arise due to injury to part of the biliary tree (biliary complications) or from procedure-related injury to other organ / systems (non-biliary complications). Biliary complications following LC have been the focus of much attention in the literature, as they lead to significant morbidity (bile collections, fistulae, jaundice, cholangitis, sepsis, and other complications of bile stasis) and usually require re-operation by a specialized surgical team, to reconstruct and drain the biliary tree adequately. On the other hand, the non-biliary injuries, although reported with a variable incidence in many series and case reports have not received their ‘due’ as complications of LC that are as dangerous and devastating as their counterparts. These injuries can range from minor to major injuries to the bowel, bladder, diaphragm or intra-abdominal / biliary vasculature and have potential to cause significant morbidity and mortality1. Bleeding complications are an important subset of these ‘non- biliary ‘ injuries and they can cause mortality on the operating table if not recognized and treated on time, they are the most frequent cause of procedure-related mortality in LC (after anaesthesia related deaths) and yet surprisingly have not been studied comprehensively despite having been reported frequently2,3.
Bleeding complications account for up to one third of all major complications seen in LC, and are the second most common cause of death in patients undergoing the procedure (after anaesthesia-related complications)2,3. The reported incidence of uncontrollable bleeding in LC can be up to 2%(reported range, 0.03% to 10%2,3,4), but the exact figure may actually be higher. Various factors may be responsible for this under-reporting, such as (a) lack of exact definition of bleeding complications; (b) many series have reported vascular injuries only but have not considered bleeding from other sites or postoperative bleeding; (c) a publication bias; (d) fear of litigation; and (e) absence of proper documentation at various centres. When bleeding occurs, the LC related mortality reportedly goes up to nearly 15%, especially when the bleeding remains unrecognized2 . In addition, in most centers routine angiography has not been used and the methods applied for diagnosis of concomitant vascular injuries vary among different centers.
Literature Review :
Literature Review :
There is a lack of uniformity in the classification of bleeding complications in LC, with various authors arbitrarily defining major and minor vascular injuries. Many have not considered the other site of bleeding that have been reported in literature4. A definition of terms and working classification was proposed by Schafer et al as follows that a bleeding complication following LC as an intraoperative bleeding complication(IBC) or a postoperative bleeding complication(PBC) based on the occurrence of a local haemorrhage either in the peritoneal cavity, the retroperitoneal space, or the abdominal wall, whereby postoperative bleeding complications occurred within the first 24 hours during the postoperative course. Each was further divided into external bleeding(abdominal wall) and internal bleeding(peritoneal cavity,retroperitoneum). Major vascular injury was defined as injury to any of the following vessels: aorta, vena cava, iliac vessels, mesenteric vessels, portal vein , splenic, omntal and renal vessels, however did not define what constituted a minor vascular injury.
Shamiyeh classification differed from Schafer in which he considered injury to the epigastric, mesenteric and omental vessels to be minor vascular injuries5. Nordesgaardt however, felt that the injury to the deep epigastric vessels should be considered a major-vessel injury, as it had the potential to cause significant morbidity and mortality6. Standardization of the definitions of bleeding in relation to LC has helped in defining the incidence of such complications in a better way, a suggested simple classification system is shown in Table 1.
Table 1 : Suggested classification of the patterns of bleeding complications in laparoscopic cholecystectomy.
Kaushik concluded that bleeding complications can be divided into major and minor depending on the need for conversion, additional surgical procedures, or blood transfusion. Thus , any bleeding that requires a laparotomy is major, irrespective of the vessel injured or the timing(intraoperative or postoperative). Similarly, any bleeding that needs additional surgical procedure(wound exploration and ligation of bleeder) or blood transfusion is also taken to be major, whereas bleeds controllable by pressure, packing; or abdominal wall hematomas that do not require any additional manoeuvres can be classified as minor bleeds7.
Various factors have been implicated in the causation of bleeding8. There seems to be no single definite cause that can be established with certainty, but the surgeon-related factors are the most important strengthening the importance of proper training and accreditation in LC (Table2) .
Table 2: Factors implicated in the causation of bleeding complications.
Surgeons who had reported less than 100 cases have been reported to have a higher complication, which had tapered off after adequate experience had been gained; this probably represent surgeons who have had some training in their first few cases and have then started operating independently8,9 .However ,this is not an absolute law, and one can face such a problem at any stage of one’s career. In fact , it has been observed that even the presence of adequate operating experience does not reduce the rate of bleeding complication, with Schafer et al. reporting a higher complications in surgeons who had operated more than 100 cases3. Various factors such as improper technique, inattentiveness, improper handling of instruments and inability to recognize the relevant anatomy contribute to the occurrence of bleeding complication at any level of experience6. Previous abdominal surgery, anatomical aberrations, adhesions and sharp dissection may also be associated with bleeding complications6. Although acute cholecystitis , cirrhosis and portal hypertension were also considered to be associated with higher complication rates and incidence of bleeding, recent reviews have suggested to the contrary; LC may actually be the procedure of choice in such patients , because of shorter operating time, lesser bleeding and lower complication rates, especially when performed by an experienced surgeon10,11,12,13.. Chao and Lee reported to perform LC on a cirrhotic patients and found that serious bleeding from a trocar site was the only trouble incurred for which the bleeding was stopped by a transmural suture technique14. Bleeding in LC can be encountered intraoperatively or in the post operative period. Intra-operative bleeding usually falls into one of the following four patterns: vessel injury, slippage of clips/ ligatures of the cystic artery, liver bed bleeding and miscellaneous6. Vessel injuries are usually the most dramatic and occur either during insertion of the first trocar or during dissection/ retraction, and were rarely seen before the advent of laparoscopic surgery. The insertion of the pnuemoperitoneum needle and the first trocar is considered by many to be the most dangerous step in LC, as it is essential a `blind step’ of the operation. As this initial step is most common to all laparoscopic operations, it has been reviewed extensively by various authors; and as mentioned earlier , the majority of bleeding complications occur in this phase of the operation14. Although the most commonly injured vessels are the epigastric15,injury can also occur to the major intra-abdominal vessels (aorta, vena cava, iliac vessels) in 0.04% to 0.18% of patients16. The distance between the abdominal wall and the great vessels can be as little as 1 to 2 cm, especially in thin individuals, contributing to the low margin of safety and the chance of aortic injury while inserting the Verres needle or the first trocar if due care is not taken. Aortic injury occurring at the time of surgery has also been reported while given skin incision with the scapel, underlining the importance of meticulous technique and care in performing each and every step of the operation. Similarly, the branching of the iliac arteries is such that the right iliac artery comes to lie just below the umbilicus, also putting it at risk of injury during forceful insertion of the trocars17, 18. Various factors have been identified in contributing to vessel injury and other trocar- related complications( Table 3)19, but as always, there seems to be no substitute for adequate surgical experience. It is also important to note that no entry technique for laparoscopy – trocar entry after creation of pneumoperitoneum, trocar entry without prior insufflations or various modifications of the open port technique is free from complications.
Table 3 : Risk factors for entry-related complications.
Although the open technique is considered by many to be safer , vascular injuries have been reported following the open technique also18. The risk of vascular injury is less for the secondry trocars, as they are placed under vision. However, bleeding from the abdominal wall(epigastric vessel) can be troublesome and can be avoided by transillumination of the abdominal wall and by observing penetration of trocars through the telescope by keeping the tip of the instruments in view throughout. The inferior epigastric vessel usually lie near the lateral border of the rectus sheath, and proper placement and direction of the trocars help in avoiding damage to them. Dissection during LC , especially within the Calot’s triangle , can also lead to a significant bleed if the right hepatic or portal vein is injured. This can happen especially when the anatomy is distorted or unrecognized, and when there is persistence in using sharp dissection in a difficult Calot’s triangle. The right hepatic is more commonly injured , but the portal vein can also be injured20,leading to a significant bleeding and the risk of biliary injury because of blind attempts to control bleeding. As regards to the impact of concomitant vascular injury to the extrahepatic biliary tree, it has been reported that the presence of vascular injury is associated with increased intraoperative bleeding during repair, more difficult reconstruction, and most importantly, high incidence of anastomotic stricture due to bile duct ischemia. Not being able to recognize the extent of injury and delaying conversion in such a situation definitely contributes to increasing morbidity and mortality of the procedure. Cases of bleeding because of slipped clips over the cystic artery and from the liver bed are also frequently encountered and can be troublesome enough to necessitate conversion to open procedure. Similarly, bleeding from parenchymal injuries to the intrabdominal organs during retraction can also be the cause of much disconcert to the operating team, forcing conversion in an otherwise successful cholecystectomy. In the postoperative period following LC , bleeding can manifest as either an internal bleeding( consequence of an intraoperatively missed vessel injury, from slipped clips/ ligatures over the cystic artery, or from the liver bed) or external bleeding( from the port sites ). Duca et al after a retrospective analysis in 9542 patients who had LC reported haemorrhage to tangential side of the cystic artery in 78 cases and total sectioning in 16 cases, in which 94 cases, laparoscopic hemostasis was achieved by clipping the artery between the lesion and it’s origin, of which only one case required conversion to open operation. He also reported that bleeding occurred from the bladder bed in 110 cases especially in those patients with acute cholecystitis and cirrhosis, in 105 patients hemostasis was achieved by using a haemostat and in the remaining 5 cases conversion was needed by suturing the peritoneum to the gall bladder21. External bleeds usually manifest after surgery, with soakage of dressings visible bleeding from port sites, and may require reoperation to salvage patients who do not respond to conservative measures. For patients having internal bleeding , it is difficult to establish the site of bleeding, and such patients may need re-exploration for its control. Surprisingly, despite there being a large volume of data on LC in literature, not much is available on the incidence of postoperative bleeding after LC, the indications for operating such patients, and the operative findings. Persisting pain, tachycardia, fall in haemoglobin and blood pressure and obtundation should alert one to the possibility of bleeding even in the absence of external bleeding, and these patients need to be evaluated carefully for their response to conservative means and the need for surgery.
Materials And Methods :
This prospective analytical study was conducted at in the Department Of Minimal Access Surgery at the World Laparoscopy Hospital, Apollo Hospital, Sir Ganga Ram Hospital and Medanta Hospital. The study included 401 patients who underwent elective laparoscopic cholecystectomy in these hospitals from January 2013 to October 2013 were studied using a designed proforma. The proforma included patient’s name (in initials), age, sex , height, weight, diagnosis, previous and duration of previous abdominal surgery, patient’s co-morbidity, International Normalized Ratio (INR), method of pneumoperitoneum, site of entry of first port, gross morphology of the liver, gall bladder status, bleeding from abdominal wall and vessels, liver bed bleeding, post-operative bleeding complication, surgical interventions and post-operative hospital stay.
Procedure :
The classical four-port technique was used for LC in the majority of cases although the three-port and single port was also used , the choice of instrument for cystic pedicle dissection and gall bladder dissection was based on the surgeon’s preference . As a routine protocol, after induction of anesthesia,a nasogastric tube is inserted and the stomach aspirated. The tube is kept in the stomach during the operation but removed at the end of the procedure. For an unscarred abdomen, the initial site of Veress needle insertion was through a vertical incision at the base of the umbilicus. For cases of previous laparotomy scars, the alternate site most commonly used was Palmer’s point located 3 cm below the left costal margin in the midclavicular line. The needle’s intraperitoneal position was confirmed by an audible double click, aspiration to rule out inadvertent bowel or vascular injury, and the saline drop test. During insufflation of carbon dioxide, a gradual rise in pressure on the insufflators, uniform abdominal distention, and obliteration of liver dullness were ensured. When an intra-abdominal pressure of 12 to 13 mmHg was achieved, the optical port is introduced routinely with a 300 telescope using a 10 mm trocar. An initial 360° scan of the entire abdomen is made primarily to exclude injury/ bleeding during the creation of the pneumoperitoneum, and secondly assess the morphology of the liver and fundus of the gall bladder. The secondary ports are then placed under direct visiualization with the laparoscope. A 10 mm trocar is then placed in the midline and left to the falciform ligament at the epigastrium. Two 5 mm ports one subcoastal trocar in the right upper quadrant and another 5 mm trocar , lower, near the right axillary line are placed. The patient is kept supine in anti- Trendelenberg position (150 head up tilt ) with left lateral tilt (15-200) for adequate exposure of the gall bladder. The dissection of the cystic pedicle was carried out by instruments using electrosurgical hook diathermy, Maryland grasper, tip of the sunction catheter and harmonic scapel as preferred by the surgeon in an orderly fashion to isolation of the cystic duct and artery. Any adhesions if present is cleared from the gall bladder using a dissector thrugh the epigastric trocar to tear the peritoneal attachments from the infundibulum. The dissection of the cystic pedicle was mostly carried out with the two hand technique starting with the anteromedial traction by left hand grasper placed on the anterior edge of Hartmann’s pouch. Once the cystic duct is visualized, the dissector is used to create a window in the triangle of Calot between the cystic duct and artery. After iiisolation of the cystic duct and artery , the clipper is introduced through the epigastric port and at least two clips are placed in the proximal side and another clip placed on the gall bladder side of the cystic then it is divided using a scissors. Similarly, the cystic artery is clipped on both ends and divided. Routine intraoperative cholangiogram was not a routine in the hospital and no record of patient in which it was carried out. The gall bladder is then retracted and separated from the liver through the areolar plane binding the gall bladder to the Glisson’s capsule linning the liver bed. This separation is done using a monopolar diathermy hook, pledget and Harmonic scapel depending on the surgeons preference. The gall bladder is then extracted through the epigastric port or umbilical port with without the use of an endobag depending on the surgeons preference. The patient is returned to the supine position and the gall bladder bed is inspected for the visualized the cystic duct and artery stump, the gall bladder bed is inspected for bleeding which is controlled with pressure packing, cauterization or spray using the monopolar diathermy hook or absorbable hemostats. The right upper quadrant is sunctioned and irrigated until clear. The use of drains was based on protocols of the individual units. The instruments and ports are then removed. The patient is reviewed within the first 6 hours for vital signs and wound dressing status, within 24-48 hours most patients are discharge.
Shamiyeh classification differed from Schafer in which he considered injury to the epigastric, mesenteric and omental vessels to be minor vascular injuries5. Nordesgaardt however, felt that the injury to the deep epigastric vessels should be considered a major-vessel injury, as it had the potential to cause significant morbidity and mortality6. Standardization of the definitions of bleeding in relation to LC has helped in defining the incidence of such complications in a better way, a suggested simple classification system is shown in Table 1.
Table 1 : Suggested classification of the patterns of bleeding complications in laparoscopic cholecystectomy.
| Major | Minor | ||
| Intra-Operative |
|
|
|
| Bleeding | Bleeding from intra-abdominal vessels:
|
Vessels —
|
|
|
|
|
Kaushik concluded that bleeding complications can be divided into major and minor depending on the need for conversion, additional surgical procedures, or blood transfusion. Thus , any bleeding that requires a laparotomy is major, irrespective of the vessel injured or the timing(intraoperative or postoperative). Similarly, any bleeding that needs additional surgical procedure(wound exploration and ligation of bleeder) or blood transfusion is also taken to be major, whereas bleeds controllable by pressure, packing; or abdominal wall hematomas that do not require any additional manoeuvres can be classified as minor bleeds7.
Various factors have been implicated in the causation of bleeding8. There seems to be no single definite cause that can be established with certainty, but the surgeon-related factors are the most important strengthening the importance of proper training and accreditation in LC (Table2) .
Table 2: Factors implicated in the causation of bleeding complications.
| Surgical Factors : | Patient-Related: | Instrument Failures: |
|
|
|
|
Surgeons who had reported less than 100 cases have been reported to have a higher complication, which had tapered off after adequate experience had been gained; this probably represent surgeons who have had some training in their first few cases and have then started operating independently8,9 .However ,this is not an absolute law, and one can face such a problem at any stage of one’s career. In fact , it has been observed that even the presence of adequate operating experience does not reduce the rate of bleeding complication, with Schafer et al. reporting a higher complications in surgeons who had operated more than 100 cases3. Various factors such as improper technique, inattentiveness, improper handling of instruments and inability to recognize the relevant anatomy contribute to the occurrence of bleeding complication at any level of experience6. Previous abdominal surgery, anatomical aberrations, adhesions and sharp dissection may also be associated with bleeding complications6. Although acute cholecystitis , cirrhosis and portal hypertension were also considered to be associated with higher complication rates and incidence of bleeding, recent reviews have suggested to the contrary; LC may actually be the procedure of choice in such patients , because of shorter operating time, lesser bleeding and lower complication rates, especially when performed by an experienced surgeon10,11,12,13.. Chao and Lee reported to perform LC on a cirrhotic patients and found that serious bleeding from a trocar site was the only trouble incurred for which the bleeding was stopped by a transmural suture technique14. Bleeding in LC can be encountered intraoperatively or in the post operative period. Intra-operative bleeding usually falls into one of the following four patterns: vessel injury, slippage of clips/ ligatures of the cystic artery, liver bed bleeding and miscellaneous6. Vessel injuries are usually the most dramatic and occur either during insertion of the first trocar or during dissection/ retraction, and were rarely seen before the advent of laparoscopic surgery. The insertion of the pnuemoperitoneum needle and the first trocar is considered by many to be the most dangerous step in LC, as it is essential a `blind step’ of the operation. As this initial step is most common to all laparoscopic operations, it has been reviewed extensively by various authors; and as mentioned earlier , the majority of bleeding complications occur in this phase of the operation14. Although the most commonly injured vessels are the epigastric15,injury can also occur to the major intra-abdominal vessels (aorta, vena cava, iliac vessels) in 0.04% to 0.18% of patients16. The distance between the abdominal wall and the great vessels can be as little as 1 to 2 cm, especially in thin individuals, contributing to the low margin of safety and the chance of aortic injury while inserting the Verres needle or the first trocar if due care is not taken. Aortic injury occurring at the time of surgery has also been reported while given skin incision with the scapel, underlining the importance of meticulous technique and care in performing each and every step of the operation. Similarly, the branching of the iliac arteries is such that the right iliac artery comes to lie just below the umbilicus, also putting it at risk of injury during forceful insertion of the trocars17, 18. Various factors have been identified in contributing to vessel injury and other trocar- related complications( Table 3)19, but as always, there seems to be no substitute for adequate surgical experience. It is also important to note that no entry technique for laparoscopy – trocar entry after creation of pneumoperitoneum, trocar entry without prior insufflations or various modifications of the open port technique is free from complications.
Table 3 : Risk factors for entry-related complications.
Surgical inexperience
|
Although the open technique is considered by many to be safer , vascular injuries have been reported following the open technique also18. The risk of vascular injury is less for the secondry trocars, as they are placed under vision. However, bleeding from the abdominal wall(epigastric vessel) can be troublesome and can be avoided by transillumination of the abdominal wall and by observing penetration of trocars through the telescope by keeping the tip of the instruments in view throughout. The inferior epigastric vessel usually lie near the lateral border of the rectus sheath, and proper placement and direction of the trocars help in avoiding damage to them. Dissection during LC , especially within the Calot’s triangle , can also lead to a significant bleed if the right hepatic or portal vein is injured. This can happen especially when the anatomy is distorted or unrecognized, and when there is persistence in using sharp dissection in a difficult Calot’s triangle. The right hepatic is more commonly injured , but the portal vein can also be injured20,leading to a significant bleeding and the risk of biliary injury because of blind attempts to control bleeding. As regards to the impact of concomitant vascular injury to the extrahepatic biliary tree, it has been reported that the presence of vascular injury is associated with increased intraoperative bleeding during repair, more difficult reconstruction, and most importantly, high incidence of anastomotic stricture due to bile duct ischemia. Not being able to recognize the extent of injury and delaying conversion in such a situation definitely contributes to increasing morbidity and mortality of the procedure. Cases of bleeding because of slipped clips over the cystic artery and from the liver bed are also frequently encountered and can be troublesome enough to necessitate conversion to open procedure. Similarly, bleeding from parenchymal injuries to the intrabdominal organs during retraction can also be the cause of much disconcert to the operating team, forcing conversion in an otherwise successful cholecystectomy. In the postoperative period following LC , bleeding can manifest as either an internal bleeding( consequence of an intraoperatively missed vessel injury, from slipped clips/ ligatures over the cystic artery, or from the liver bed) or external bleeding( from the port sites ). Duca et al after a retrospective analysis in 9542 patients who had LC reported haemorrhage to tangential side of the cystic artery in 78 cases and total sectioning in 16 cases, in which 94 cases, laparoscopic hemostasis was achieved by clipping the artery between the lesion and it’s origin, of which only one case required conversion to open operation. He also reported that bleeding occurred from the bladder bed in 110 cases especially in those patients with acute cholecystitis and cirrhosis, in 105 patients hemostasis was achieved by using a haemostat and in the remaining 5 cases conversion was needed by suturing the peritoneum to the gall bladder21. External bleeds usually manifest after surgery, with soakage of dressings visible bleeding from port sites, and may require reoperation to salvage patients who do not respond to conservative measures. For patients having internal bleeding , it is difficult to establish the site of bleeding, and such patients may need re-exploration for its control. Surprisingly, despite there being a large volume of data on LC in literature, not much is available on the incidence of postoperative bleeding after LC, the indications for operating such patients, and the operative findings. Persisting pain, tachycardia, fall in haemoglobin and blood pressure and obtundation should alert one to the possibility of bleeding even in the absence of external bleeding, and these patients need to be evaluated carefully for their response to conservative means and the need for surgery.
Materials And Methods :
This prospective analytical study was conducted at in the Department Of Minimal Access Surgery at the World Laparoscopy Hospital, Apollo Hospital, Sir Ganga Ram Hospital and Medanta Hospital. The study included 401 patients who underwent elective laparoscopic cholecystectomy in these hospitals from January 2013 to October 2013 were studied using a designed proforma. The proforma included patient’s name (in initials), age, sex , height, weight, diagnosis, previous and duration of previous abdominal surgery, patient’s co-morbidity, International Normalized Ratio (INR), method of pneumoperitoneum, site of entry of first port, gross morphology of the liver, gall bladder status, bleeding from abdominal wall and vessels, liver bed bleeding, post-operative bleeding complication, surgical interventions and post-operative hospital stay.
Procedure :
The classical four-port technique was used for LC in the majority of cases although the three-port and single port was also used , the choice of instrument for cystic pedicle dissection and gall bladder dissection was based on the surgeon’s preference . As a routine protocol, after induction of anesthesia,a nasogastric tube is inserted and the stomach aspirated. The tube is kept in the stomach during the operation but removed at the end of the procedure. For an unscarred abdomen, the initial site of Veress needle insertion was through a vertical incision at the base of the umbilicus. For cases of previous laparotomy scars, the alternate site most commonly used was Palmer’s point located 3 cm below the left costal margin in the midclavicular line. The needle’s intraperitoneal position was confirmed by an audible double click, aspiration to rule out inadvertent bowel or vascular injury, and the saline drop test. During insufflation of carbon dioxide, a gradual rise in pressure on the insufflators, uniform abdominal distention, and obliteration of liver dullness were ensured. When an intra-abdominal pressure of 12 to 13 mmHg was achieved, the optical port is introduced routinely with a 300 telescope using a 10 mm trocar. An initial 360° scan of the entire abdomen is made primarily to exclude injury/ bleeding during the creation of the pneumoperitoneum, and secondly assess the morphology of the liver and fundus of the gall bladder. The secondary ports are then placed under direct visiualization with the laparoscope. A 10 mm trocar is then placed in the midline and left to the falciform ligament at the epigastrium. Two 5 mm ports one subcoastal trocar in the right upper quadrant and another 5 mm trocar , lower, near the right axillary line are placed. The patient is kept supine in anti- Trendelenberg position (150 head up tilt ) with left lateral tilt (15-200) for adequate exposure of the gall bladder. The dissection of the cystic pedicle was carried out by instruments using electrosurgical hook diathermy, Maryland grasper, tip of the sunction catheter and harmonic scapel as preferred by the surgeon in an orderly fashion to isolation of the cystic duct and artery. Any adhesions if present is cleared from the gall bladder using a dissector thrugh the epigastric trocar to tear the peritoneal attachments from the infundibulum. The dissection of the cystic pedicle was mostly carried out with the two hand technique starting with the anteromedial traction by left hand grasper placed on the anterior edge of Hartmann’s pouch. Once the cystic duct is visualized, the dissector is used to create a window in the triangle of Calot between the cystic duct and artery. After iiisolation of the cystic duct and artery , the clipper is introduced through the epigastric port and at least two clips are placed in the proximal side and another clip placed on the gall bladder side of the cystic then it is divided using a scissors. Similarly, the cystic artery is clipped on both ends and divided. Routine intraoperative cholangiogram was not a routine in the hospital and no record of patient in which it was carried out. The gall bladder is then retracted and separated from the liver through the areolar plane binding the gall bladder to the Glisson’s capsule linning the liver bed. This separation is done using a monopolar diathermy hook, pledget and Harmonic scapel depending on the surgeons preference. The gall bladder is then extracted through the epigastric port or umbilical port with without the use of an endobag depending on the surgeons preference. The patient is returned to the supine position and the gall bladder bed is inspected for the visualized the cystic duct and artery stump, the gall bladder bed is inspected for bleeding which is controlled with pressure packing, cauterization or spray using the monopolar diathermy hook or absorbable hemostats. The right upper quadrant is sunctioned and irrigated until clear. The use of drains was based on protocols of the individual units. The instruments and ports are then removed. The patient is reviewed within the first 6 hours for vital signs and wound dressing status, within 24-48 hours most patients are discharge.